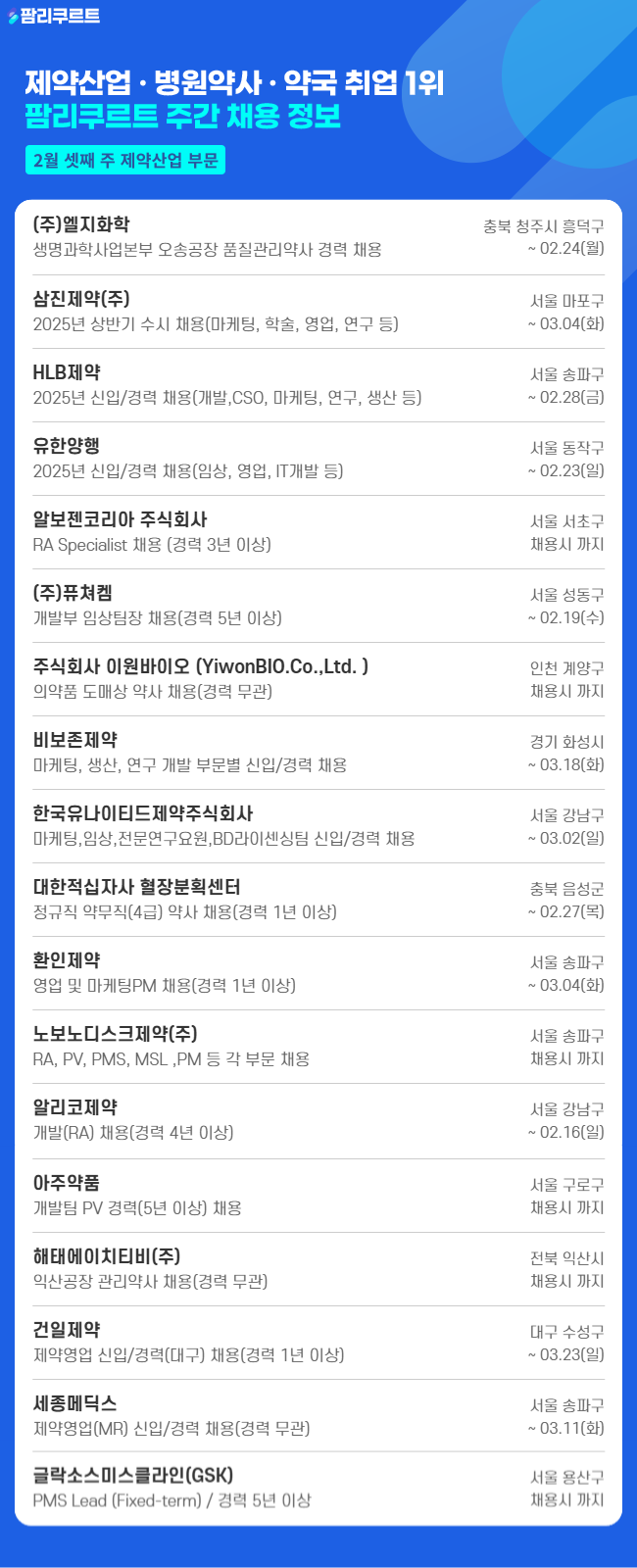
[팜리쿠르트] 유한양행·삼진제약·노보노디스크 등 채용
- 손형민
- 2025-02-18 06:15:27
-
가
- 가
- 가
- 가
- 가
- 가
- AD
- 겨울을 이기는 습관! 피지오머 스프레이&젯노즐에 대한 약사님들의 생각은?
- 이벤트 바로가기
손형민(shm@dailypharm.com)
Copyright ⓒ 데일리팜. All rights reserved. 무단 전재 및 재배포 금지.
- 익명 댓글
- 실명 댓글
- 댓글 0
- 최신순
- 찬성순
- 반대순
운영규칙
오늘의 TOP 10
- 1부산 창고형약국, 서울 진출?...700평 규모 개설 준비
- 25년 엔트레스토 분쟁 종지부...제네릭 승소 이끈 3대 쟁점
- 3국내제약 16곳, '린버크' 결정형특허 분쟁 1심 승리
- 4차바이오, 카카오·LG와 동맹...'3세 경영' 협업 전략 가동
- 5수제트리진, 새로운 기전의 비마약성 진통제
- 6R&D·공정 다시 짠다…제약사별로 갈린 AI 활용 지도
- 7'이모튼', 약국당 180T 균등 공급...19일부터 신청
- 8SK케미칼, 트루셋 저용량 쌍둥이약 허가…2031년까지 독점
- 9한국파마, CNS 외형 반등…디지털헬스로 확장 모색
- 10미국, 의약품 품목관세 조치 임박…관세율·범위 촉각





















































![[신신] 아렉스 두번효과로 강력한](https://cdn.platpharm.co.kr/2025/10/2510230254510000664.webp)
![[리쥬올]레티노 멜라세럼 저자극 레티놀](https://cdn.platpharm.co.kr/2025/09/2509260219360000145.webp)
![[SK케미칼] 속편한정 복합소화제](https://cdn.platpharm.co.kr/2025/12/2512040916400005920.webp)
![[유한양행] 콘택콜드 걸렸구나 생각되면](https://cdn.platpharm.co.kr/2025/10/2510282252420008436.webp)
![[종근당] 벤포벨에스 어른들의 피로회복제](https://cdn.platpharm.co.kr/2025/07/2507290841210004645.webp)
![[유한양행] 미녹펜겔 탈모스팟 집중케어](https://cdn.platpharm.co.kr/2025/09/2509220824180004563.webp)
![[더본메디칼] ATC인쇄리본 특가](https://cdn.platpharm.co.kr/2025/04/2504100527360001454.jpg)

![[신신] 새사래 상처연고 습윤밴드](https://cdn.platpharm.co.kr/2025/10/2510210339570001784.webp)
![[SK케미칼] 트라스트패취 피록시캄 성분](https://cdn.platpharm.co.kr/2025/10/2510020656150002375.webp)
![[셀로맥스] 베베락스 온가족 안심 관장약](https://cdn.platpharm.co.kr/2025/09/2509171131320018843.webp)
![[리쥬올]리쥬올 PDRN 약국 1위 PDRN](https://cdn.platpharm.co.kr/2025/09/2509260220180000170.webp)
![[레킷코리아] 목 아플 때, 스트렙실 허니&레몬 트로키 12정](https://i.baropharm.com/products/202502/1739520767049.png?label=PLAN_01)
![[켄뷰] 오리지널 폼타입, 로게인5%폼에어로졸60g](https://i.baropharm.com/products/dc84d96e-d0b4-46bc-bcc8-d62016406fe4.png)
![[휴온스 ] 비듬을 한번에, 니조랄 2%액](https://i.baropharm.com/products/478a284d-4361-4b4a-8a00-8bab80f34319.png?label=PLAN_01)
![[아워팜] 아이들이 먼저찾는, 바로타민 kids 미네랄](https://i.baropharm.com/products/202512/1766121243228.png)
![[켄뷰] 다양한 통증에, 타이레놀정 500mg 10정](https://i.baropharm.com/products/6c6ea4f4-7ab2-44f2-a165-f062d80f525b.png)
![[아워팜] 건강한 힘, 바로바이오틱스 kids 비피더스 50억](https://i.baropharm.com/products/202511/1763106585375.png)
![[아워팜] CJ웰케어, 바이오코어 1000억 유산균](https://i.baropharm.com/products/202512/1765955416559.png)
![[아워팜] 에너지 바로 충전, 바로콤](https://i.baropharm.com/products/202512/1764922282624.png)
![[아워팜] 혈행건강 히어로, 혈행건강 초임계 rTG 오메가3 프리미엄](https://i.baropharm.com/products/f724f191-7052-4ad5-bc92-cda889cf13a6.png)
![[아워팜] 우리아이 맞춤설계, 바로타민 kids 엘더베리맛](https://i.baropharm.com/products/8eae7689-61be-4b71-9608-05364b05ea12.png)
![[오펠라] 부드럽고 편안한, 둘코락스에스장용정 20정](https://i.baropharm.com/products/202511/1762260404625.png)
![[한독] 붙이는 통증 전문가, 케토톱 액티브 플라스타(쿨) 40매](https://i.baropharm.com/products/202503/1741829602305.png)



