- LOGIN
- MemberShip
- 2025-12-18 08:18:02
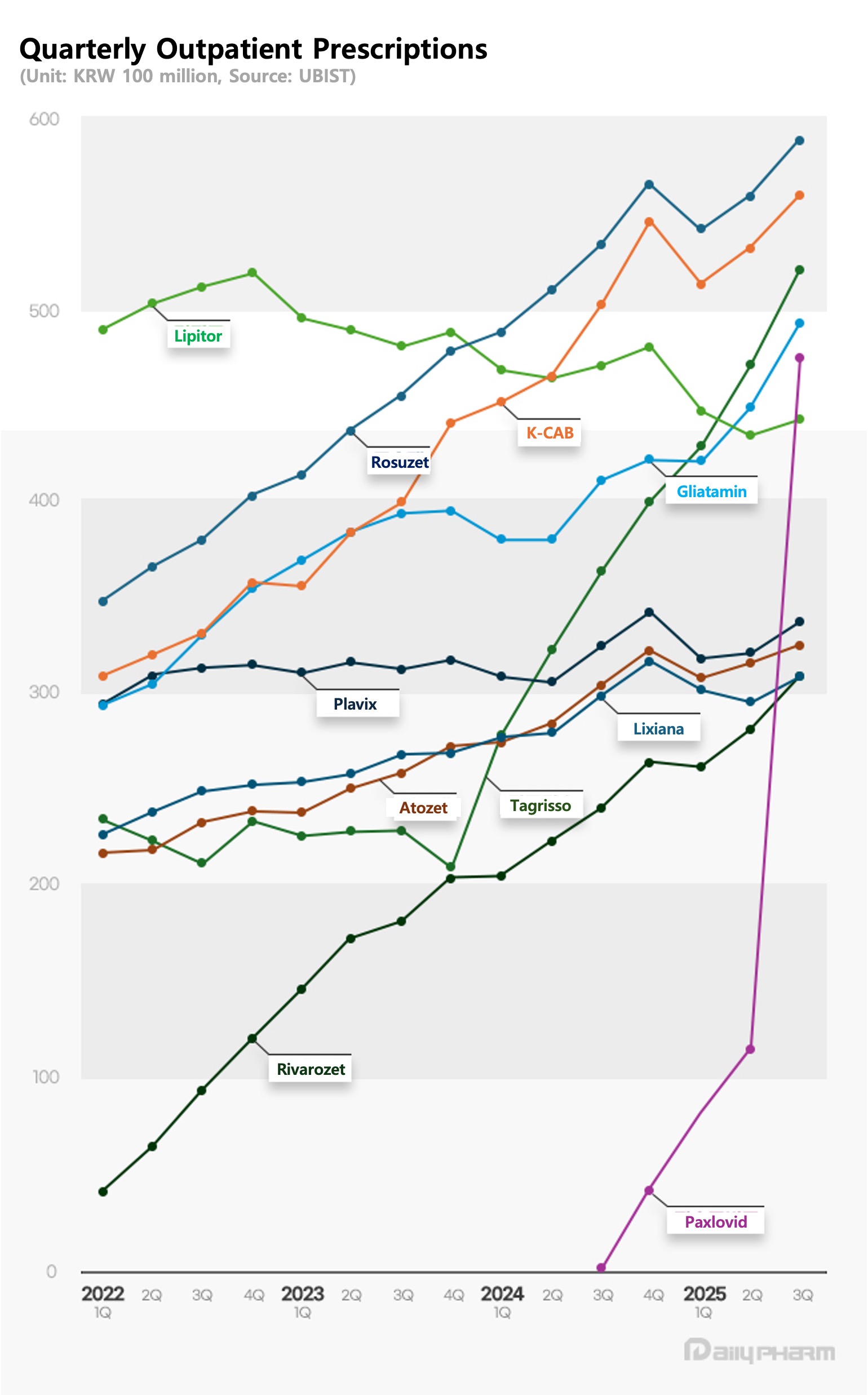
- Rosuzet and K-CAB maintain dual lead…Paxlovid sales surge
- by Chon, Seung-Hyun | translator Alice Kang | 2025-10-17 06:18:20
Homegrown drugs continue to dominate the top ranks of the outpatient prescription market.
Quarterly outpatient prescriptions of the new combination therapy Rosuzet and the new drug K-CAB both surpassed KRW 50 billion, firmly maintaining a two-top system.
Meanwhile, Livalozet continued its steep growth trajectory, and Paxlovid saw a sharp rise in prescriptions following its reimbursement listing.
According to the pharmaceutical research institution UBIST, Hanmi Pharmaceutical's hyperlipidemia combination drug Rosuzet recorded the highest outpatient prescription sales of KRW 58.9 billion in the third quarter.
Rosuzet’s prescriptions grew 11.0% compared to the third quarter of last year and increased 5.3% from the previous quarter.
1 position for seven consecutive quarters.
From 2021 to 2023, it ranked No.
2 overall and topped the annual outpatient prescription sales for the first time last year.
Launched in late 2015, Rosuzet is a fixed-dose combination of rosuvastatin and ezetimibe.
Statin-ezetimibe combinations are seeing soaring demand in prescribing practices due to their excellent efficacy in lowering low-density lipoprotein cholesterol and their relatively low cost burden.
Since 2020, Rosuzet has consistently generated over KRW 100 billion in annual prescriptions, reaching KRW 210.3 billion in 2023, and became the first domestically developed drug ever to exceed KRW 200 billion in annual prescriptions.
Cumulative sales for the first three quarters of 2025 reached KRW 169.2 billion, up 10.2% year-on-year, making another over KRW 200 billion a year record highly likely.
HK Inno.N’s new drug K-CAB ranked second with KRW 56.1 billion in Q3 prescriptions, up 11.4% year-on-year.
Its cumulative total for the first three quarters reached KRW 160.8 billion, a 13.1% increase versus the same period last year.
Approved in 2018 as Korea's 30th domestically developed new drug, K-CAB is a ‘Potassium-Competitive Acid Blocker (P-CAB)’ for treating gastroesophageal reflux disease (GERD).
Its mechanism of action involves competitively binding to the proton pump and potassium ions at the final stage of acid secretion in gastric wall cells, thereby inhibiting gastric acid secretion.
K-CAB has sequentially secured 5 more indications, including erosive and non-erosive GERD, gastric ulcers, H.
pylori eradication in combination with antibiotics for peptic or chronic atrophic gastritis, and maintenance therapy after GERD treatment.
With its rapid onset of action and dosing flexibility of use regardless of meals, K-CAB continues to post robust growth.
It first exceeded KRW 100 billion in 2021, its third year on the market, and has now achieved over KRW 100 billion annually for four consecutive years.
Having already surpassed KRW 100 billion in the first half of this year, K-CAB is poised to break the KRW 200 billion mark for 2025.
JW Pharmaceutical’s dyslipidemia combination drug Livalozet posted KRW 31.0 billion in Q3, up 29.0% year-on-year, ranking ninth overall.
Livalozet contains pitavastatin and ezetimibe.
Launched in October 2021, Livalozet has maintained steep growth since its release.
Its prescription surged in 2022 with sales of KRW 31.8 billion, soaring to KRW 93.3 billion in both 2023 and last year.
With cumulative third-quarter prescriptions reaching KRW 85.3 billion, a 27.7% year-on-year increase, Livalozet is poised to surpass KRW 100 billion this year.
Pfizer’s COVID-19 treatment Paxlovid ranked fifth overall with KRW 47.7 billion in outpatient prescriptions for Q3 alone.
Paxlovid is an oral antiviral that inhibits replication of the COVID-19 virus and is primarily prescribed to high-risk patients at risk of severe disease progression.
Initially supplied free of charge by the government, the drug transitioned to general hospital prescriptions after public procurement ended in mid-2024.
Since October last year, Paxlovid has fully entered the prescription market following its inclusion in the National Health Insurance reimbursement list.
The maximum insurance price ceiling was set at KRW 941,940, with the patient's copayment set at 5%.
Paxlovid made its full debut in the prescription market in the fourth quarter of last year, generating KRW 4.1 billion in prescription sales.
In the second quarter of this year, it recorded KRW 11.4 billion in prescription sales, surpassing KRW 10 billion, and in the third quarter, it jumped more than fourfold compared to the previous quarter.
Paxlovid's cumulative prescription sales for the third quarter reached KRW 67.3 billion.
This rapid surge is attributed to the sharp increase in COVID-19 patients combined with Paxlovid's high price point.
Paxlovid surpassed KRW 10 billion in monthly prescription sales for the first time in August, with KRW 17.4 billion, and last month it topped the overall market with KRW 24.9 billion, shaking up the entire prescription landscape.
AstraZeneca's anticancer drug Tagrisso saw its third-quarter outpatient prescription amount reach KRW 52.2 billion, a 43.0% increase year-on-year.
Its cumulative prescription performance for the third quarter was KRW 142.4 billion, a 47.2% increase.
Tagrisso is an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI).
EGFR-TKIs are targeted anticancer drugs prescribed to patients with metastatic non-small cell lung cancer (NSCLC) harboring EGFR mutations.
Since last year, Tagrisso, along with Yuhan Corporation's Leclaza, has had its reimbursement expanded to include ‘first-line treatment for locally advanced or metastatic non-small cell lung cancer with specific genetic mutations’.
While anticancer drugs are predominantly prescribed to hospitalized patients, Tagrisso's oral formulation has significantly boosted its outpatient prescription volume.
Tagrisso's prescription sales surged from KRW 21 billion in Q4 2023 to KRW 32.3 billion in Q1 last year, maintaining its upward trajectory to exceed KRW 50 billion in Q3 this year.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









