- LOGIN
- MemberShip
- 2025-12-21 04:01:02
- Good pharma companies will receive better drug price
- by Lee, Jeong-Hwan | translator Kim, Jung-Ju | 2024-02-08 05:49:27
The government will promote innovation in the pharmaceutical industry by overhauling the existing system that only provides preferential treatment – a drug price at the highest level among its substitutes - for companies that supply essential drugs or receive a new drug approval for the drug for the first time in Korea.
It will also continue expanding insurance coverage through measures such as reflecting the value of innovative new drugs that demonstrated high therapeutic effects in severe and rare diseases in the drug price, and expediting and expanding the number of targets that benefit from health insurance coverage.
Furthermore, the government plans to prepare measures for drug price reevaluations, high-priced drug management, and advancing the generic drug pricing structure.
These are the contents of the 2nd Comprehensive National Health Insurance Plan (2024-2028) that was released by the Ministry of Health and Welfare on February 4th.
Basically, the ministry will strengthen economic support using health insurance finances for pharmaceutical companies that have made achievements in innovating healthcare, health security, and national economic development.
The changes were made as part of the government’s efforts to create a system that reflects the growing public and social demand for innovative medical technologies that provide new treatment opportunities for patients with severe and incurable diseases or are more cost-effective than existing technologies.
In terms of health security, the government plans to support the establishment of a basic infrastructure to enable the domestic supply of essential medicines that are directly related to the life and health of the people so that the drugs can be stably produced in Korea even if there is a disruption in the overseas supply chain.
Pharmaceutical companies that contribute through drug innovation, or drive national development, etc.
will benefit from higher drug prices The Ministry of Health and Welfare will improve policies to strengthen access to innovative new drugs.
First, it will continue to expand coverage for highly effective severe and rare disease treatments.
The order of the reimbursement listing will be prioritized based on disease severity, availability of alternative drugs, superiority of treatment, cost-effectiveness, and financial burden.
It will also continue to expedite health insurance listing of new drugs for life-threatening diseases.
The approval, evaluation, and negotiation linkage system that has been implemented will reduce the time required for health insurance reimbursement from 330 days to 150 days.
To be eligible for the parallel review system, the drug has to have no alternative and be a treatment for a life-threatening disease, demonstrate a significant improvement in clinical effect compared to existing drugs, and be approved for fast-track review by the MFDS.
The MOHW will also expand eligibility for expediting listings.
Currently, the expedited listing system is being piloted in 2 rare pediatric disease groups - neuroblastoma and hereditary cholestatic stasis – based on which the scope of diseases eligible for fast-track registration will be expanded from 2025.
New drugs that are recognized as innovative will be able to receive expedited listing support by recognizing their economic feasibility even if their incremental cost-effectiveness ratio (ICER) exceeds a certain level.
The drug pricing premiums provided for each pharmaceutical company will also be expanded.
The MOHW believes that it is necessary to calculate drug prices differently based on the company’s degree of contribution to improving public health, health sustainability, and national economic development.
Therefore, the ministry will prepare a mechanism that provides preferential drug prices to pharmaceutical companies that have led healthcare innovation and contributed to building a stable supply chain by investing in R&D for new and incrementally modified drugs, supplying essential drugs, and creating jobs.
Currently, only companies that supply essential medicines that received a new drug approval for the drug for the first time in Korea receive a preferential price at the highest price level of its alternatives.
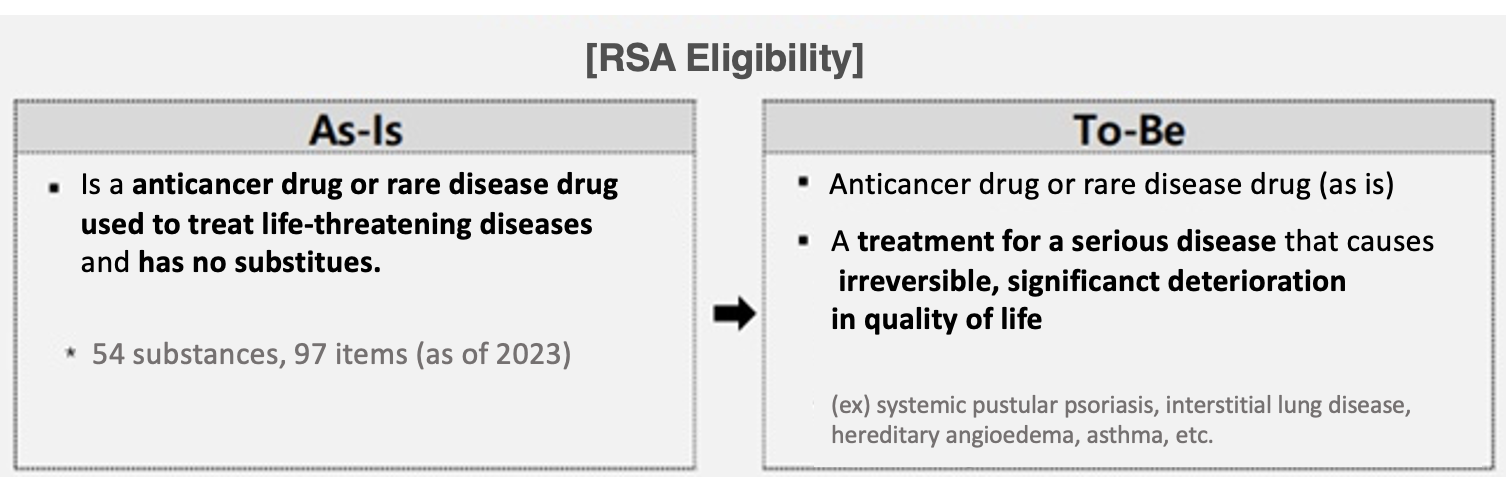
The government will improve patient access to drugs by expanding risk-sharing system (RSA) treatments for serious diseases that cause significant and irreversible deterioration in quality of life.

The price of national essential drugs made with domestic ingredients will receive preferential treatment.
First, in the case of newly designated national essential medicines, this drug will be given preferential drug prices over other generics when the main ingredient of the generic drug is produced domestically.
Drug prices will also be raised quickly to resolve the issue of unstable drug supply.
The MOHW is preparing a procedure to quickly raise drug prices of drugs that have become difficult to produce due to rising costs that arose due to their unstable supply and demand after COVID-19.
By simplifying the review process based on the criteria for adjusting the insurance price ceiling and concurrently progressing with the NHIS drug pricing negotiations, the MOHW plans to reduce the time required for drug price increases from '210 days+@' to '30 days+@.’ In addition, the MOHW will continue to improve the adequacy of compensation for drug shortage prevention drugs by reflecting manufacturing costs.
It will expand the scope of national essential medicines that are eligible for drug price increases and differentiate support to specific classifications.
The upper limit for Korean herbal medicine formulations will also be adjusted.
Considering the rising cost of APIs and strengthened manufacturing and quality control regulations, the government will consider raising the insurance price ceiling for herbal medicines based on the results of a field survey.
Will reduce drug expenditures through drug price reevaluations, management of high-priced drugs, restructuring of generics, etc. The MOHW will establish an integrated price adjustment mechanism for reasonable drug cost management.
First, it will prepare a mid to long-term strategic plan to unify the currently fragmented mechanisms for adjusting the price ceiling.
Starting this year, the ministry will conduct policy research on establishing a mid- to long-term strategy.
The MOHW will also review patent-expired drugs and adjust the price if their domestic price is higher than their highest price in foreign countries.
In the case of reevaluating patent-expired drugs, the government plans to start with chronic disease drugs that have a large number of generics.
However, drugs that require a stable supply, such as antidepressants, will not be subject to reevaluations.
In addition, the management of high-priced drugs for serious diseases will be strengthened.
To reduce the patient’s burden of medical expenses as well as the government’s health insurance finances, various types of risk-sharing systems, including outcome-based reimbursement, will be applied to newly listed drugs.
The MOHW will also strengthen follow-up management of high-priced drugs for serious diseases based on patient safety and medical effects.
It will also rationalize the price-volume agreement system, improving such as increasing the price cut rate for drugs that have a high claims amount or expanding the subjects that can be excluded from being subject to PVA.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










