- LOGIN
- MemberShip
- 2025-12-17 22:22:48
- Launch of K-new drugs in the Chinese market is accelerated
- by Chon, Seung-Hyun | translator Hong, Ji Yeon | 2025-09-10 06:12:21
New drugs developed in Korea are successively entering the Chinese market.
Based on their commercial success in the Korean market, new P-CAB (Potassium-Competitive Acid Blocker) drugs for gastroesophageal reflux disease are quickly entering the approximately KRW 3 trillion-worth Chinese market.
Fexuclue has become the second P-CAB to enter the Chinese market after K-CAB.
Zaqubo is also in the approval process.
Improvement is expected in the massive pharmaceutical trade deficit with China, which has exceeded KRW 5 trillion over the past 10 years.
Fexuclue Launches in the Chinese Market, following K-CAB...Zaqubo Also Enters Approval Process According to industry sources on September 8, Daewoong Pharmaceutical received product approval for Fexuclue 40mg from China's National Medical Products Administration (NMPA) on September 5.
Fexuclue obtained approval in China for the treatment of gastroesophageal reflux disease.

Fexuclue is a 'P-CAB' gastroesophageal reflux disease treatment developed by Daewoong Pharmaceutical.
It was approved as the 34th domestically developed new drug in December 2021.
Fexuclue is a new Korean drug that Daewoong Pharmaceutical successfully developed with proprietary technology over 13 years, starting in 2008.
P-CAB anti-ulcer drugs work by competitively binding to the proton pump and potassium ions, which are located in the final stage of acid secretion in parietal cells, thereby inhibiting gastric acid secretion.
P-CAB new drugs have proven their commercial value in Korea by proven advantages such as a faster onset of action and the ability to be taken regardless of meals, unlike conventional PPI (Proton Pump Inhibitor) class products.
According to global market research firm IMS data, the size of China's anti-ulcer drug market is approximately KRW 3 trillion, making it the largest in the world.
With its 1.4 billion population adopting more Westernized dietary habits, the number of gastroesophageal reflux disease patients is rapidly increasing, and treatment demand is expected to expand.
Daewoong Pharmaceutical projected, "Fexuclue is expected to rapidly increase market share in the Chinese anti-ulcer drug market by improving upon the drawbacks of existing PPIs, such as slow onset of action, short half-life, and the need for pre-meal administration." Fexuclue is highly regarded for its long half-life, which enables sustained acid suppression and provides excellent relief for nocturnal heartburn.
Among drugs in the same class, Fexuclue is the only one to have clinically proven its effect in 'alleviating chronic cough' caused by acid reflux.
Daewoong Pharmaceutical has set the second half of 2026 as the goal for Fexuclue's launch.
The company plans to deploy a full-scale market entry strategy that reflects the characteristics and demand of the local Chinese market.
Park Seong-soo, CEO of Daewoong Pharmaceutical, said, "This Chinese product approval will be a very important turning point for Fexuclue's leap to becoming a global blockbuster drug." Park added, "We will strive to ensure that Fexuclue becomes the most trusted treatment option for patients and medical professionals in China, the world's largest anti-ulcer drug market." Fexuclue is the second Korea-developed new P-CAB drug to enter the Chinese market, following K-CAB.
In April 2022, HK inno.N's K-CAB was approved in China for the treatment of erosive esophagitis.
The local product name was decided as 'Taisinzan (泰欣赞),' meaning 'carrying great joy.' HK inno.N pursued K-CAB's overseas expansion in 2015 by signing a technology export agreement with Chinese pharmaceutical company Luoxin Group and passed the Chinese hurdle in seven years.
The agreement with Luoxin Group includes a total of $18.5 million in milestone payments based on upfront payment, clinical development, approval, and commercialization stages.
K-CAB has been approved for three indications in China: erosive esophagitis, duodenal ulcer, and Helicobacter pylori eradication therapy.
Since January of this year, K-CAB's scope of reimbursement has been expanded with the addition of the duodenal ulcer indication to the China's National Reimbursement Drug List (NRDL).

In March 2023, Onconic Therapeutics signed a technology export agreement for Zaqubo with Chinese pharmaceutical company Livzon Pharmaceutical Group.
The contract value is up to $127.5 million.
Onconic Therapeutics received a non-refundable upfront payment of $15 million and is set to receive up to $112.5 million in technology fees based on development, approval, and commercialization milestones.
Livzon Pharmaceutical Group began patient dosing for Zaqubo's Chinese Phase 3 clinical trial at the end of last year and successfully completed the trial last month, submitting a product approval application to the China's National Medical Products Administration.
Expectations are high for the success of Korea-developed new P-CAB drugs in the Chinese market, building on their commercial success in the Korean market.
In the Korean market, K-CAB's prescription sales surpassed KRW 100 billion in 2021, its third year since launch, and has recorded over KRW 100 billion in prescription sales for four consecutive years.
In the first half of this year, its prescription sales increased by 14.0% year-on-year to KRW 104.7 billion, possibly leading to an annual prescription sales of KRW 200 billion.
Fexuclue, launched in Korea in July 2022, recorded KRW 12.9 billion in prescription sales in its first year, and its sales soared over six times in two years, reaching KRW 78.8 billion last year.
Its prescription amount in the first half of this year was KRW 43.2 billion, a 22.5% increase from the same period last year.
Zaqubo, which entered the prescription market in earnest after receiving health insurance reimbursement in October of last year, recorded KRW 17.2 billion in outpatient prescription sales in the first half of this year.
Last year's pharmaceutical trade deficit with China amounted to KRW 630B...Expectation for improving 'deficit of KRW 5T over 10 years' If Korea-developed new P-CAB drugs achieve commercial success in China, an improvement in the trade balance with China is also expected.
The performance of Korean companies' pharmaceuticals in the Chinese market has been low.
According to the Ministry of Food and Drug Safety, the value of pharmaceutical exports to China last year was $407.27 million, less than half of the import value of $865.61 million.
Last year's pharmaceutical trade deficit with China amounted to $457.34 million (approximately KRW 630 billion).
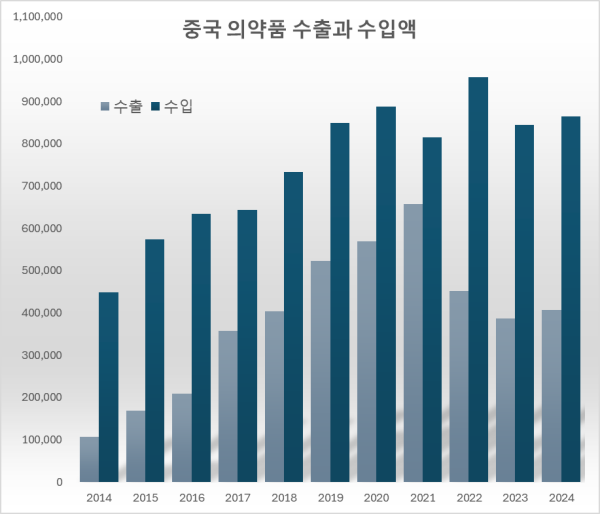
Light Blue-Exports, Blue-Imports Over the past 10 years, from 2015 to last year, the total pharmaceutical trade deficit with China reached $3.66939 billion (approximately KRW 5.1 trillion).
The pharmaceutical trade deficit with China expanded by $115.78 million over the past 10 years, from $341.56 million in 2014.
During this period, pharmaceutical exports to China increased by $299.88 million, from $107.39 million to $407.27 million.
However, imports increased by a significantly larger amount, $415.67 million, from $448.94 million to $864.61 million.
This sluggish performance in exporting finished pharmaceutical products from Korea, coupled with the accelerated penetration of Chinese raw materials into the Korean market, is identified as a key factor in the worsening trade balance.
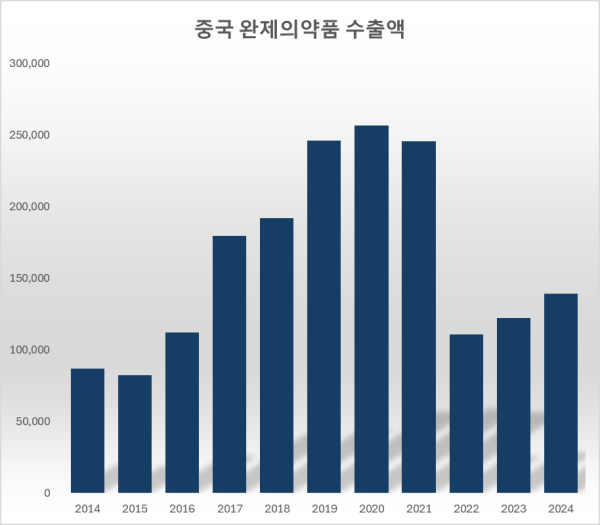
Last year, the value of finished pharmaceutical product exports to China was $138.98 million.
While this represents a 59.7% increase over 10 years from $87.01 million in 2014, it is a 45.8% decrease compared to the $256.44 million recorded in 2020.
The slow progress of Korean companies in the Chinese market is pointed out as a reason for the worsening trade balance.
In contrast, imports of Chinese active pharmaceutical ingredients (APIs) reached $816.32 million last year, a 110.2% jump from $388.31 million 10 years ago.
In 2014, China was the 6th largest country of origin for pharmaceutical imports, but it has now jumped to the 3rd position last year.
In 2014, China was the second-largest country in terms of pharmaceutical exports, following Japan, but it fell to 9th place last year.
An industry official said, "The continuous decrease in the cost structure of finished pharmaceutical products due to the government's sustained drug price reduction policy inevitably leads to a higher demand for cheaper imported APIs for cost reduction," and added, "If Korean companies' efforts into entering the Chinese finished pharmaceutical product market are initiated, an improvement in the trade balance can be expected."
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










