- LOGIN
- MemberShip
- 2025-12-21 16:46:58
- Paxlovid and Dong-A’s Diosmin Powder were approved in July
- by Lee, Hye-Kyung | translator Kim, Jung-Ju | 2023-08-07 05:23:10
The number of prescription drug approvals decreased significantly in July.
The approvals, which had continuously increased from 70 in May to 93 in June, had fallen to record 29 in July.
However, the drugs approved were nevertheless as significant as Pfizer Korea’s COVID-19 treatment ‘Paxlovid (nirmatrelvir, ritonavir)’, which had first been introduced to Korea through the EUA (emergency use authorization) during the COVID-19 outbreak, received formal approval as a new drug in Korea, and the Takeda-developed Celltrion-owned ‘Alo Gliptinpio Tab’ was approved for export.

Compared to the previous month, the ETC approvals were reduced by 64, and OTC approvals increased to 47 and exceeded the number of ETC approvals.
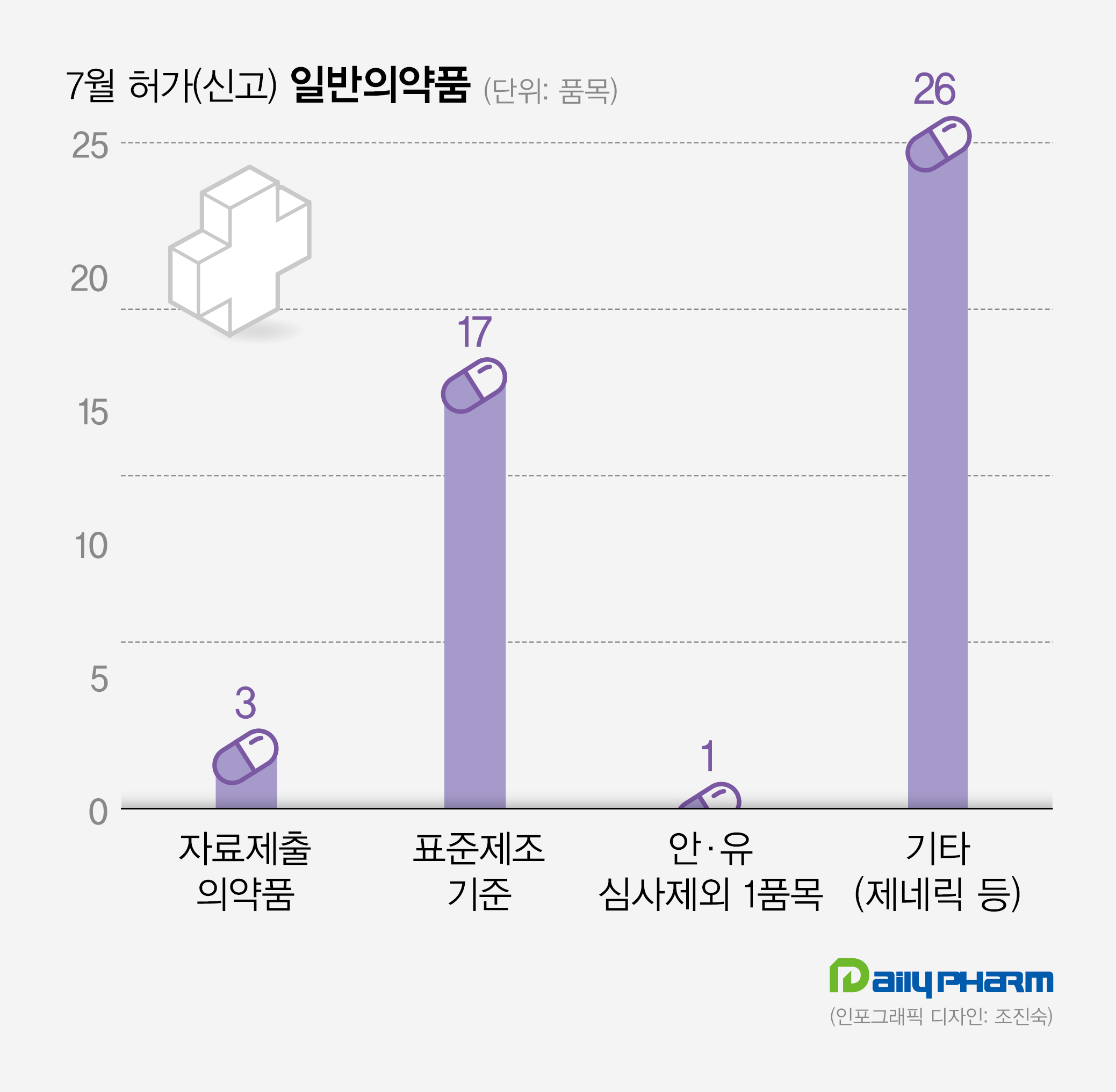
◆OTC drugs== A total of 47 over-the-counter drugs were approved (registered) in July.
Among those, 3 were data submission drugs(incrementally modified drugs, IMDs), which are modified versions of existing drugs that underwent safety and efficacy reviews due to changes in ingredient, salt base, or dosage form.
The Gnal-N series was first released in 2009 with the ibuprofen combo ‘Gnal-N Tab,’ followed by ‘Gnal-N Q Tab', ‘Gnal-N Nose Soft Cap, ‘Gnal-N Cold Soft Cap,’ ‘Gnal-N Cough Soft Cap,’ ‘Gnal-N Nose Plus Soft Cap.’ The company received additional approval for ‘Gnal-N Ace Soft Cap’ which contains a combination of acetaminophen and riboflavin on July 12, and increased its Gnal-N product line-up to 13.
Gnal-N Ace Soft Cap is indicated for headache, toothache, pain after tooth extraction, sore throat, ear pain, joint pain, neuralgia, back pain, muscle pain, shoulder pain (stiff shoulder), bruise pain, fracture pain, sprain pain, menstrual pain.
analgesia of traumatic pain, chills, and fever.

Each packet of the drug contains diosmin 600mg that can be dissolved into water for intake.
It is indicated to improve symptoms related to venous insufficiency (leg heaviness, pain), as supplementary treatment for disorders caused by capillary fragility, and treatment of symptoms related to hemorrhoids.
It will be released to pharmacies as a box with 10 packets.
Meanwhile, latecomers such as Dong-A Pharmaceutical, Hanmi Pharm, Chodang Pharm, and Samjin Pharm have increased their presence in the diosmin market by releasing high-dose oral hemorrhoids treatments one after another.
Dong-A Pharmaceutical's annual sales of Diomax Tab doubled from KRW 500 million in 2020 to KRW 1 billion in 2021, based on IQVIA data.
Last year, Diomax posted sales of KRW 1.1 billion, occupying 48% of the market the high-dose (600mg) oral hemorrhoids treatment market.
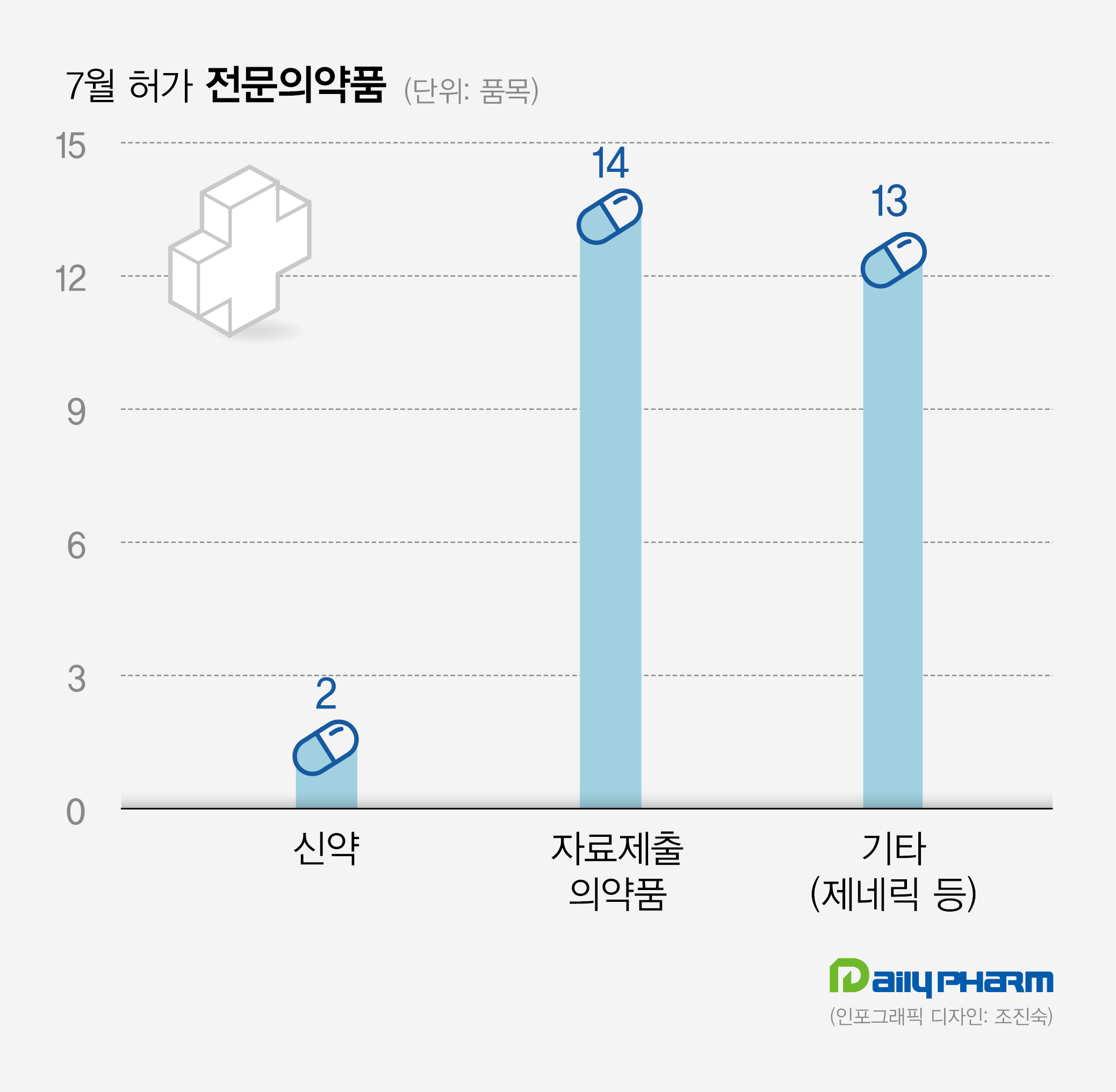
14 data submission drugs and 13 generics and others were also approved the same month.
Celltrion’s ‘Celltrion Alo Gliptinpio Tab 25·15mg, 25·30mg (for export, Approved on July 6 and 7) Celltrion received approval for Celltrion Alo Gliptinpio Tab 25·15mg, and 25·30mg for export on July 6 and 7, respectively.
The drug is a new combination made by the company using its rights for ‘Nesina' and 'Actos', which it acquired from the Japanese pharmaceutical company Takeda Pharmaceuticals.
Takeda Pharmaceuticals had been directly selling Nesina in Korea until 2020, but in December 2020, it sold all rights, including sales rights and patents, of 12 ETC drug brands and 6 OTC drugs in 9 Asia-Pacific countries to Celltrion Pharm to adjust the size of its debt.
I did.
Alogliptin and pioglitazone can help patients control blood sugar levels, and patients with Type 2 diabetes can take them to control blood sugar along with diet and exercise.
Since last year, Celltrion Pharm has been strengthening its product lineup receiving approval for drugs to export overseas.
Sanofi-Aventis Korea’s Enjaymo Inj (new drug, Approved on July 12) Sanofi’s ‘Enjaymo Inj,’ which is indicated to treat adults with cold agglutinin disease (CAD), was also recently approved in Korea.
The drug is used to treat the breakdown of red blood cells (hemolysis) in adults with cold agglutinin disease (CAD), which is a form of autoimmune hemolytic anemia (AIHA), CAD is a rare type of autoimmune hemolytic anemia caused when antibodies called cold agglutinins bind to the surface of red blood cells.
When cold agglutinins bind to the surface of red blood cells, the body's immune system mistakenly attacks and destroys healthy red blood cells.
Pfizer Korea’s Paxlovid Tab (new drug, Approved on July 14) Pfizer Korea’s ‘Paxlovid,’ which was first introduced in Korea through the emergency use authorization (EAU) track during the spread of COVID-19, received official approval in Korea.
Paxlovid was granted EAU by the MFDS on December 27, 2021, the same year the number of confirmed and seriously ill patients with COVID-19 surged and the Omicron variant spread, raising the need for an oral treatment for COVID-19.
It has been formally approved as a prescription drug 1 year and 7 months after its introduction to Korea.
Patients who take Paxlovid take two tablets at the same time.
Nirmatrelvir blocks protease (3CL protease) to prevent the production of proteins necessary for viral replication, thereby inhibiting the proliferation of the virus, and ritonavir inhibits the enzyme (CYP3A4) that breaks down nirmatrelvir, extending its duration of effect.
After a careful review of the results of the Phase III clinical trial (therapeutic confirmatory trial) conducted on adult patients, the drug was officially approved in Korea in accordance with the 'Pharmaceutical Affairs Act,’ The EAU for Paxlovid, which has allowed patients to use the drug free of charge, will remain in place as is.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










