- LOGIN
- MemberShip
- 2025-12-18 05:15:53
- Only Vemlidy’s sales grew in the ₩300B HBV drug mkt
- by Kim, Jin-Gu | translator Alice Kang | 2025-08-14 06:13:28
Korea's hepatitis B treatment market, which is valued at KRW 300 billion annually, is rapidly restructuring around Vemlidy (tenofovir alafenamide).
Among original products, only Vemlidy is maintaining its growth, and in the generic market, only the Vemlidy series has shown a significant increase in sales.
HBV treatment market reaches KRW 150.9 billion in 1H, up 2% year-on-year According to the pharmaceutical market research institution UBIST on the 13th, the domestic hepatitis B treatment market reached KRW 150.9 billion in the first half of this year.
This represents a 2% increase from the KRW 147.6 billion in the first half of last year.
At this pace, the HBV drug market is expected to record prescription sales of over KRW 300 billion for the second consecutive year.
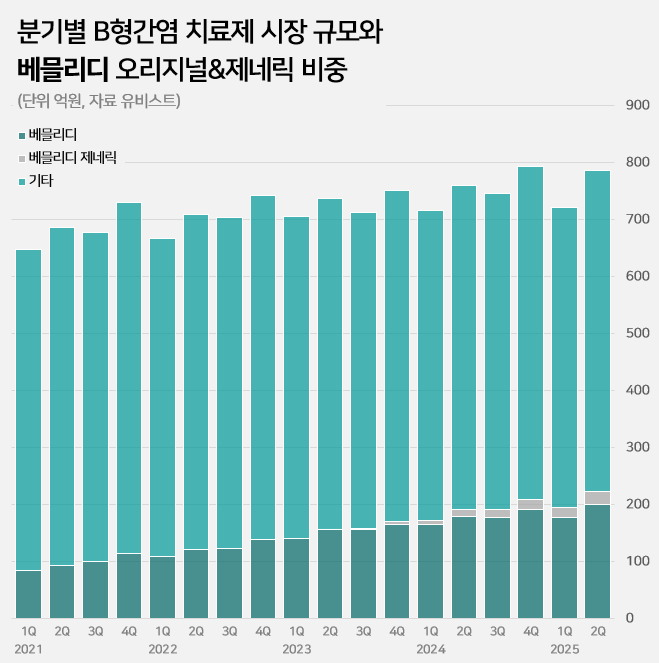
The market had exceeded KRW 300 billion in 2018 but decreased to KRW 273.1 billion in 2019.
This was due to the expiration of the patent for Gilead Sciences' Viread, which had been leading the market, and the subsequent price reduction of the drug.
The market further shrank to KRW 266 billion in 2020.
Since 2021, the market has shifted to growth.
In 2021, the market increased by 4% to KRW 275.6 billion.
In 2022, it reached KRW 283.8 billion, then grew by 3% for two consecutive years to KRW 283.8 billion in 2022, then KRW 292.3 billion in 2023.
Last year, it expanded further to over KRW 300 billion.
Vemlidy becomes the only original drug to show growth…grows 10% in one year Gilead's Vemlidy drove market growth.
Vemlidy’s first-half prescription sales reached KRW 37.8 billion, a 10% increase from the previous year.
Vemlidy is a new hepatitis B drug developed by Gilead as a successor to Viread.
While the existing Viread is highly effective in suppressing the hepatitis B virus, its drawbacks include side effects such as renal dysfunction and reduced bone density.
Vemlidy overcomes these limitations of Viread.
In clinical trials, no such side effects were observed.
Given the challenging nature of hepatitis B, which is difficult to cure, the long-term safety of Vemlidy has emerged as a key advantage.
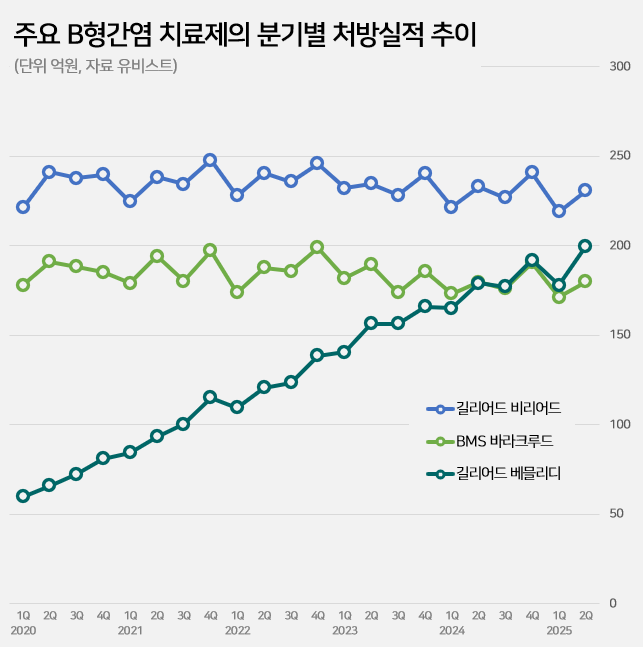
Meanwhile, the market leader, Viread (tenofovir sofosbuvir), saw a 1% decrease from KRW 45.4 billion to KRW 44.9 billion in one year.
Sales of BMS's ‘Baraclude (entecavir)’ decreased by 1% from KRW 35.3 billion to KRW 35.1 billion.
In addition, ‘Zeffix (lamivudine)’, ‘Besivo (besifovir)’, ‘Levovir (clevudine)’, and ‘Sebivo (telbivudine)’ all saw a decrease in prescriptions.
Hepsera (adefovir) has been completely withdrawn from the market since 2023.
Among the major products, while Viread and Baraclude saw a slowdown, Vemlidy was the only one to show an increase in its prescription sales, leading to changes in the market rankings.
Vemlidy ranked third in the market behind Viread and Baraclude until the first half of last year, but then surpassed Baraclude to move up to second place.
The gap with Viread narrowed from KRW 11 billion to KRW 7.2 billion in just one year.
Generic market also sees rapid growth of Vemlidy follow-on drugs...
accelerates market restructuring The same trend has been observed in the generic market.
Vemlidy generics are the only ones that showed continued growth.
The combined prescription amount for Vemlidy generics in the first half of this year was KRW 4.1 billion, more than double the KRW 2 billion recorded in the same period last year.
Although it showed a somewhat sluggish trend initially after its official launch in February 2023, its growth has accelerated since last year.
Sales of Samil Pharmaceutical's ‘Vemlino’ increased from KRW 9 billion in the first half of last year to KRW 18 billion in the first half of this year, while sales of Dong-A ST's ‘Vemlia’ increased from KRW 7 billion to KRW 13 billion, each more than doubling in sales.
The remaining products also saw their prescription sales increase by more than twofold.
On the other hand, Baraclude generics and Viread generics have seen a slowdown in their upward trend.
The combined prescription sales of Baraclude generics decreased by 3% from KRW 16.3 billion to KRW 15.8 billion over the past year.
Sales of Viread generics also decreased by 3% from KRW 7.2 billion to KRW 7 billion.
The industry expects that the market restructuring centered on Vemlidy will accelerate in both the original and generic markets.
This is because Bemliride has already been recommended as a first-line treatment by liver associations in the US, Europe, and South Korea.
In South Korea, its reimbursement has been approved for the treatment of non-cirrhotic liver cirrhosis and liver cancer since 2022.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










