- LOGIN
- MemberShip
- 2025-12-18 04:51:49
- Mounjaro set for launch in Korea this month
- by Son, Hyung Min | translator Hong, Ji Yeon | 2025-08-08 06:02:37
Eli Lilly Korea's diabetes and obesity treatment, Mounjaro, is set to launch in mid-August.
Therefore, it will compete against Novo Nordisk's Wegovy.
Lilly plans to handle Mounjaro's distribution directly, a strategy that differentiates it from Novo Nordisk, which is currently seeking co-promotion partners.
According to the distribution industry on August 8, Lilly Korea plans to launch Mounjaro in Korea this month.
The company has organized its own sales and marketing teams and is entering the Korean market through its existing network of distributors.
The launch is expected to take place in the third week of August.
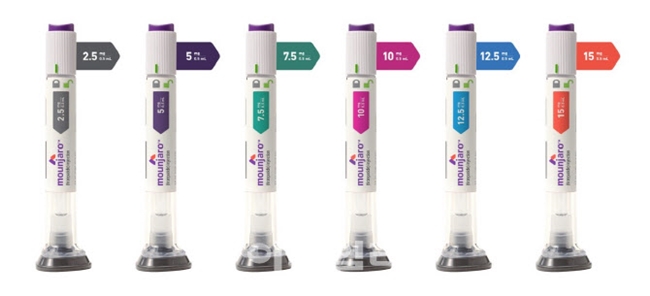
The domestic supply price for Mounjaro is reportedly around KRW 278,000 for the 2.5mg dose, KRW 369,000 for the 5mg dose, and KRW 521,377 for the 7.5mg and 10mg doses.
The initial launch will include only the 2.5mg and 5mg formulations.
Mounjaro is a once-weekly subcutaneous injection.
Treatment begins with a 2.5mg dose, with the dosage increasing every four weeks.
The maximum dose is 15mg.
An official from Lilly Korea stated, "We are preparing for the launch of Mounjaro in Korea in the third week of August.
We plan to release only the 2.5mg and 5mg doses initially, but we will supply higher-dose formulations, including 7.5mg, 10mg, 12.5mg, and 15mg, without disruption to meet patient demand."

Lilly Korea has recently begun contract discussions with over 30 pharmaceutical distributors, including the largest domestic distributor, Ji-O-Young.
These negotiations are reportedly in the final stages, with discussions underway with 12 to 15 distributors in the Seoul metropolitan area and 10 to 14 in other regions.
For Mounjaro's distribution, Lilly Korea has selected a model in which distributors directly supply healthcare institutions.
The company will only be involved in setting the supply price, leaving the actual transaction price to the discretion of the distributors.
However, a specific price guide has been established to ensure the product isn't sold "too expensively or too cheaply." Having prior experience in supplying GLP-1 drugs, such as Trulicity, in Korea, Lilly Korea believes it can maintain its competitive edge with this direct distribution model.
Novo Nordisk, on the other hand, currently entrusts the nationwide distribution of Wegovy to a foreign-based pharmaceutical distributor, Zuellig Pharma, with supplies also handled by different distributors, such as Bluemtech.
Recent reports suggest that Novo Nordisk and domestic pharmaceutical company Chong Kun Dang may enter a co-promotion agreement for Wegovy.
Chong Kun Dang has previous experience selling the obesity drug Qsymia from Alvogen Korea.
However, according to the company, no final decision has been made.
The market is focused on how much Mounjaro will impact Wegovy's dominance.
Even with delays in its official domestic launch, Wegovy rapidly captured the market through various channels.
According to market research firm IQVIA, Wegovy's sales in the first quarter of this year reached KRW 79.4 billion, accounting for a 73.2% share of the total obesity drug market.
Wegovy, a GLP-1 monotherapy containing semaglutide, gained immediate attention following its launch in Korea in October 2024.
Despite its high price, demand for prescriptions surged due to its significant weight loss effects.
Wegovy quickly rose to the top of the obesity drug market with sales of KRW 60.3 billion in the fourth quarter of last year.
The obesity drug market size in the third quarter of last year was KRW 47.4 billion, but it skyrocketed by 97.9% to KRW 93.8 billion in just one quarter with the launch of Wegovy.
Mounjaro Demonstrates Superior Weight Loss Effectiveness Over Wegovy Mounjaro is a new diabetes drug developed by Lilly.
This treatment, a GIP/GLP-1 receptor dual agonist, works by acting on both Glucose-dependent Insulinotropic Polypeptide (GIP) receptors and Glucagon-like Peptide-1 (GLP-1) receptors.
This mechanism promotes insulin secretion, improves insulin resistance, and reduces glucagon secretion, resulting in a decrease in blood sugar levels before and after meal.
Major causes of reduced incretin in diabetic and obese patients are decreased GLP-1 secretion and impaired GIP insulin-stimulating effect.
GLP-1 and GIP are hormones responsible for two-thirds of the post-meal insulin response.
It is also approved for chronic weight management in obese adults (initial BMI ≥ 30 kg/m²) or overweight adults (initial BMI ≥ 27 kg/m² to < 30 kg/m²) with at least one weight-related comorbidity (e.g., hypertension, dyslipidemia, type 2 diabetes, obstructive sleep apnea, or cardiovascular disease), as an adjunct to a reduced-calorie diet and increased physical activity.
Mounjaro has the advantage of not only controlling blood sugar but also demonstrating excellent weight loss effects.
Mounjaro proved its weight loss efficacy through the Phase 3 SURMOUNT-1 clinical trial, conducted in obese or overweight adults with at least one comorbidity but without diabetes.
Notably, Mounjaro demonstrated superior weight loss effects in SURMOUNT-5, a direct comparative clinical trial against Wegovy.
The results of the Phase 3 trial showed that the mean weight loss percentage at 72 weeks for the Mounjaro treatment group (10mg or 15mg) was 20.2%, which was a greater improvement compared to the 13.7% for the Wegovy treatment group (1.7mg or 2.4mg).
Having confirmed its weight loss effects in clinical trials for Mounjaro, Lilly launched Zepbound, an obesity treatment with the same active ingredient, in the U.S.
market in November 2023.
In Korea, both the diabetes and obesity indications will be marketed under the single product name, Mounjaro.
Lilly Korea plans to apply for insurance reimbursement for its Mounjaro indication for type 2 diabetes.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










