- LOGIN
- MemberShip
- 2025-12-18 04:51:49
- Donepezil ↑8%, memantine 10%↑…replaces choline alfoscerat
- by Kim, Jin-Gu | translator Alice Kang | 2025-08-07 06:10:41

In the first half of this year, donepezil’s sales recorded KRW 162.6 billion, an 8% increase from the previous year.
Sales of memantine-based formulation increased by 10% year-on-year, and nicergoline-based formulations also increased by 36%.
Prescription market for the choline alfoscerate alternative ‘donepezil’ grows from KRW 151.1 billion to KRW 162.6 billion in one year According to the pharmaceutical market research institution UBIST on the 6th, the outpatient prescription sales of donepezil-based formulations in the first half of this year amounted to KRW 162.6 billion.
This represents an 8% increase from the KRW 151.1 billion in the first half of last year.
Donepezil is used to treat symptoms of Alzheimer's dementia.
The original product is Hanok's Aricept.
In August 2000, Daewoong Pharmaceutical imported the finished product from the original developer and began domestic production and supply.
Subsequently, the domestic marketing rights were transferred to Hanok.
Sales are handled by Eisai Korea.
In 2019, the indication for ‘vascular dementia’ was removed following a clinical reevaluation, but it has had little impact on prescription sales.
On the contrary, it has continued to grow by around 5-8% annually since 2020.
Prescription sales of donepezil-based formulations increased from KRW 243.6 billion in 2020 to KRW 259.8 billion in 2021, KRW 171.5 billion in 2022, KRW 291.9 billion in 2023, and to KRW 313.9 billion last year.
Considering the upward trend in prescriptions in the first half of this year, its sales are expected to continue to increase at the same level as previous years.
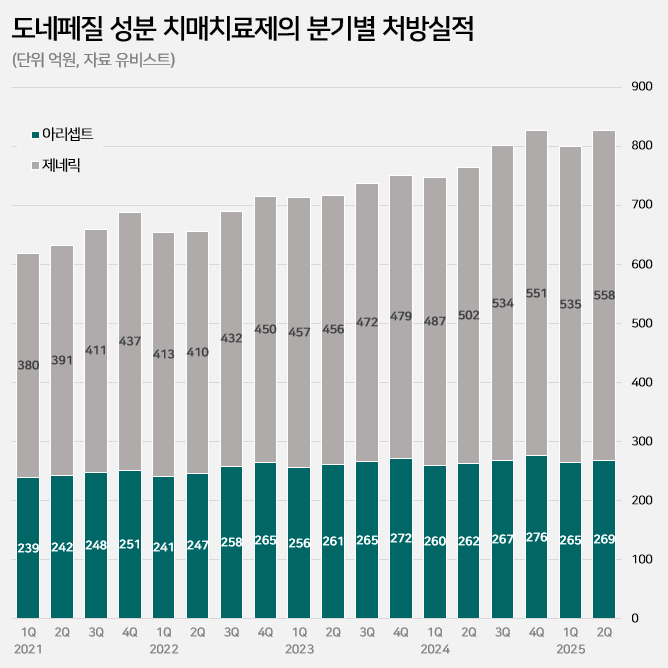
In particular, sales of generic products have shown marked growth.
While the sales of the original product Aricept increased by only 2% from KRW 52.2 billion in the first half of last year to KRW 53.4 billion in the first half of this year, sales of generic products increased by 11% from KRW 98.9 billion to KRW 109.3 billion during the same period.
Sales of Daewoong Bio's Beacept increased 14% from KRW 14.9 billion to KRW 17 billion, Samjin Pharmaceutical's Neutoin increased 27% to KRW 6.4 billion in a year, and Whan In Pharm’s Donegil increased 88% to KRW 3.8 billion.
The rise of donepezil preparations is analyzed to connected to the crisis of choline alfoscerate products.
Choline alfoscerate, which were previously the most widely used in the field of dementia prevention, are facing market withdrawal due to reduced reimbursement for their indications and clinical reevaluations.
Initially, choline alfoscerate drugs had three indications: ▲secondary symptoms and degenerative or degenerative brain syndrome caused by cerebrovascular defects; ▲emotional and behavioral changes; ▲senile pseudo-depression.
During the clinical reevaluation process, two of the three indications were removed, excluding its use as a treatment of ‘secondary symptoms and degenerative or degenerative brain disorders caused by cerebral vascular defects.’ A separate clinical reevaluation to verify the drug’s safety and efficacy is also underway.
If pharmaceutical companies fail to prove efficacy in the clinical reevaluation process, their drugs will have to be completely withdrawn from the market.
In addition, they must return 20% of the prescription amounts accrued during the clinical trial period to the health authorities.
Given this situation, the pharmaceutical industry has been intent on finding alternatives to replace choline alfoscerate.
In this process, donepezil, which has similar indications to choline alfoscerate, has emerged as one of the main alternatives.
Although it has been used consistently for dementia prevention, there has been a series of new product approvals since the threat of choline preparations being withdrawn from the market.
In fact, since June 2020, when the Ministry of Food and Drug Safety requested companies with choline alfoscerate-based products to submit clinical trial data, 39 pharmaceutical companies have received new approvals for 50 donepezil preparations.
Choline alfoscerate-based formulations, which were previously the most widely used ingredient in the field of dementia p #Sales of memantine-based formulations rise from KRW 27.7 billion to KRW 30.4 billion in one year...
Sales of nicergoline preparations also jumped 36% The same situation goes for memantine and nisergoline-based products.
These two ingredients are considered major alternatives to donepezil and choline alfoscerate.
Prescription of memantine-based products increased 10% in the first half of this year, reaching KRW 30.4 billion, compared to the KRW 27,7 billion in the same period last year.
Memantine is indicated for the treatment of moderate-to-severe Alzheimer's disease.
The original product is Lundbeck's ‘Ebixa,’ which was approved in 2003.
Like donepezil-based formulations, new product approvals have skyrocketed since June 2020.
Twenty-seven pharmaceutical companies have been approved for 37 products in the last five years.
One in three of all memantine products (109) on the market has been approved since the controversy arose over the efficacy of choline preparations.
At the end of last year, approvals for new combination products that contain memantine and donepezil followed one after another.
After HyundaiPharm received approval for ‘DM Duo,’ eight companies obtained approval for 14 additional products.
Related products collectively achieved prescription sales of KRW 500 million in the first half of this year.
Among choline esterase inhibitor alternatives, nicergoline-based formulations have had the most new product approvals in the industry.
Nicergoline is approved for the ‘primary treatment of dementia symptoms such as memory impairment, concentration disorders, judgment disorders, and lack of initiative associated with primary degenerative vascular dementia and mixed dementia.’ The original product is Ildong Pharmaceutical’s Sermion.
Ildong Pharmaceutical received approval for this product in 1997.
Until 2022, no subsequent products were approved, excluding those for export.
However, since 2023, new product approvals have been granted one after another.
Following Hanmi Pharmaceutical's approval of ‘Nicegoline’ in January 2023, 39 companies have received approval for 53 products as of recently.
Prescription sales of nicergoline formulations increased by 36% from KRW 3.3 billion in the first half of last year to KRW 4.5 billion in the first half of this year.
In particular, the growth of new follow-on products that were recently released to the market has been rapid.
The combined prescription sales of the follow on products, which amounted to only KRW 400 million in the first half of last year, increased more than threefold to KRW 1.5 billion in just one year.
Sermion’s sales also increased slightly from KRW 2.9 billion to KRW 3 billion.
Sales of existing choline preparations recorded KRW 294 billion in the first half of this year.
Although this represents a slight decrease from the KRW 301.4 billion in the first half of last year, it still boasts prescription sales of nearly KRW 300 billion, demonstrating its continued popularity.
Daewoong Bio and Chong Kun Dang, which hold the top two positions in the existing choline alfoscerate market, are also actively seeking alternatives.
Daewoong Bio's ‘Gliatamin’ and Chong Kun Dang's ‘Chongkundang Gliatirin’ accounted for more than half of the total choline preparation market in the first half of the year.
In preparation for the withdrawal of its choline alofscerate products, Daewoong Bio has received approval for ‘Beacept,’ which contains donepezil, ‘Glibixa’ containing memantine, and ‘Daewoong Nicergoline’ containing nicergoline.
Chong Kun Dang has obtained approval for ‘Neuromanthyn’ containing memantine and ‘Nexcholine’ containing nicergoline.
Additionally, at the end of last year, it added ‘Neurocept Duo,’ a combination of donepezil and memantine.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










