- LOGIN
- MemberShip
- 2025-12-18 05:15:53
- Lixiana solely leads DOAC mkt… Xarelto, Eliquis↓
- by Kim, Jin-Gu | translator Alice Kang | 2025-07-30 06:16:09

Prescription sales of Lixiana (edoxaban) increased by 8% year-on-year, solidifying its lead in Korea’s market.
Its market share in the DOAC market expanded to 49%, and the analysis is that Lixiana will continue its lead in the market until the expiration of its substance patent next year.
Eliquis (apixaban) and Xarelto (rivaroxaban) showed sluggish performance.
Lixiana saw a 30% decline in prescriptions due to price reductions following the launch of generics in the fourth quarter of last year.
Xarelto, whose patent expired earlier, has also shown a clear downward trend in sales in recent years.
DOAC prescriptions in the first half of the year reach KRW 122.4 billion...Lixiana solidifies sole lead in the market According to the pharmaceutical market research institute UBIST on the 28th, the domestic DOAC market prescription volume in the first half of this year was KRW 122.4 billion.
This is a 4% decrease from the KRW 127.1 billion it had rendered in the first half of last year.
DOACs are anticoagulants that prevent blood clots by directly acting on blood coagulation factors.
They have replaced warfarin, which inhibits vitamin K metabolism, and are increasingly being used in clinical practice.
In Korea, Xarelto was approved in 2009, followed by Pradaxa and Eliquis in 2011, and Lixiana in 2015.
When the product first appeared, it was commonly referred to as NOAC (New Oral Anticoagulant), but as it has been more than 10 years since its initial approval, it is now called DOAC (Direct Oral Anticoagulant), referring to its mechanism of action that directly acts on coagulation factors.
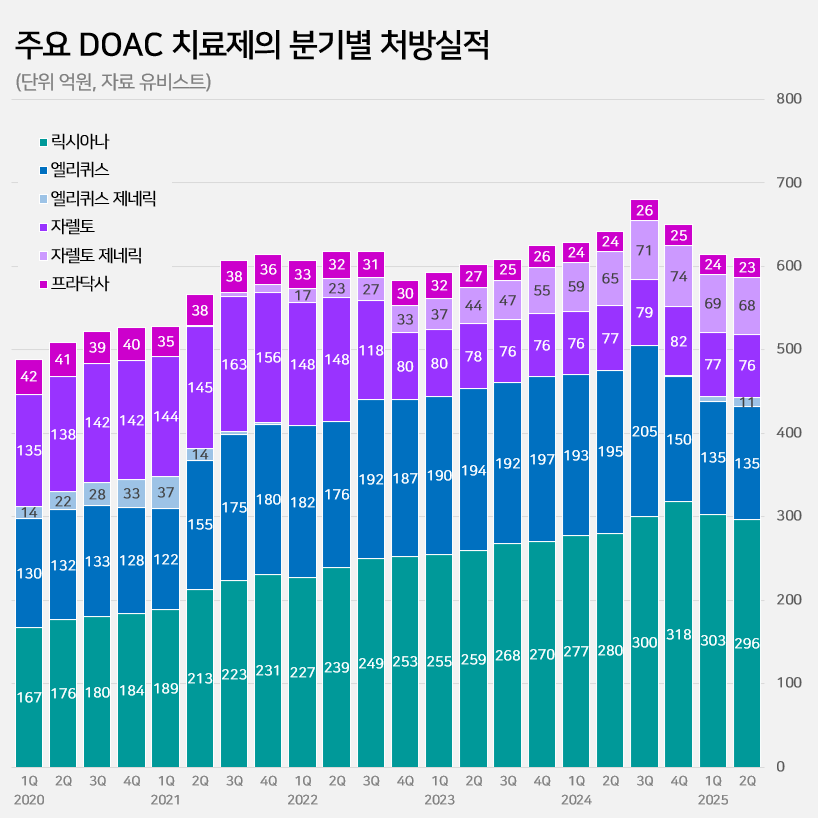
Lixiana’s prescription sales in the first half of this year reached KRW 59.9 billion, an 8% increase from the KRW 55.7 billion in the same period last year.
Lixiana was the last DOAC to be released, but the drug quickly increased its prescription sales and has maintained its market leadership since 2019.
With an annual growth of around 10%, prescriptions rose from KRW 60.4 billion in 2019 to KRW 117.5 billion last year, nearly doubling in five years.
Its share in the total DOAC market expanded to 49% in the first half of this year.
This means that Lixiana accounts for nearly half of the KRW 260 billion in the DOAC market.
The industry expects the market dominance to continue until the end of next year.
The substance patent for Lixiana will expire in November next year.
About 20 domestic pharmaceutical companies are expected to release generic versions at that time.
Currently, 13 companies have been approved to sell generic versions of Lixiana, including Nexpharm Korea, Dong-A ST, Samsung Pharm, Shinil Pharmaceutical, Shinpoong Pharm, Anguk Pharmaceutical, Ildong Pharmaceutical, Genuone Science, Union Korea Pharm, Korea Prime Pharm, Hutecs Korea Pharmaceutical, and Handok Pharm.
In addition, Samjin Pharmaceutical, HLB Pharmaceutical, Theragen Etex, and DongKwang Pharm have filed for an invalidation trial (passive scope confirmation trial) regarding the Lixiana formulation patent.
If they win the first trial, they will be able to release generic versions in line with the expiration of the substance patent, like other companies.
Furthermore, Alico Pharmaceutical and Korean Drug have been approved to conduct bioequivalence tests for the release of their generic versions of Lixiana.
Eliquis·Xarelto sales in clear decline…due to the release of generic versions and drug price cuts On the other hand, Eliquis and Xarelto are in a clear downward trend.
The sales decline of both products is analyzed to be affected by patent expirations and the subsequent entry of generics.
Eliquis sales fell 30% from KRW 38.8 billion in the first half of last year to KRW 27.1 billion in the first half of this year.
This is due to the impact of price reductions caused by the entry of generics.
Eliquis’ price was reduced by 30% in September last year.
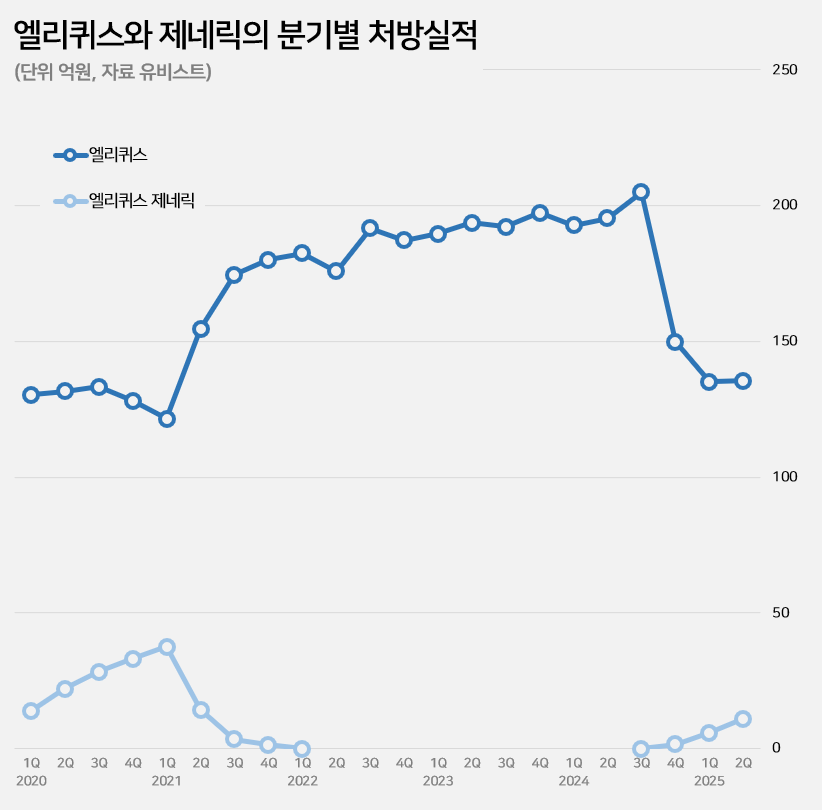
Eliquis generics were originally released in June 2019.
At that time, generic companies released their products based on the first and second instance rulings in favor of them in patent litigation.
However, the situation reversed in April 2021 when the Supreme Court overturned the first and second court rulings.
The generic drugs were immediately withdrawn from the market.
Following the expiration of Eliquis' substance patent in September last year, the generics returned to the market after three and a half years.
Eliquis generics have been expanding prescriptions since their return to the market, reaching KRW 200 million in the fourth quarter of last year, KRW 600 million in the first quarter of this year, and then KRW 1.1 billion in the second quarter.
By product, the situation is similar to that before the generics withdrew from the market three and a half years ago.
At the time, the top two generic products in terms of prescription sales, Chong Kun Dang’s ‘Liquisia’ and Samjin Pharmaceutical's ‘Elxaban,’ have maintained their positions as the top two generics even after re-entering the market.
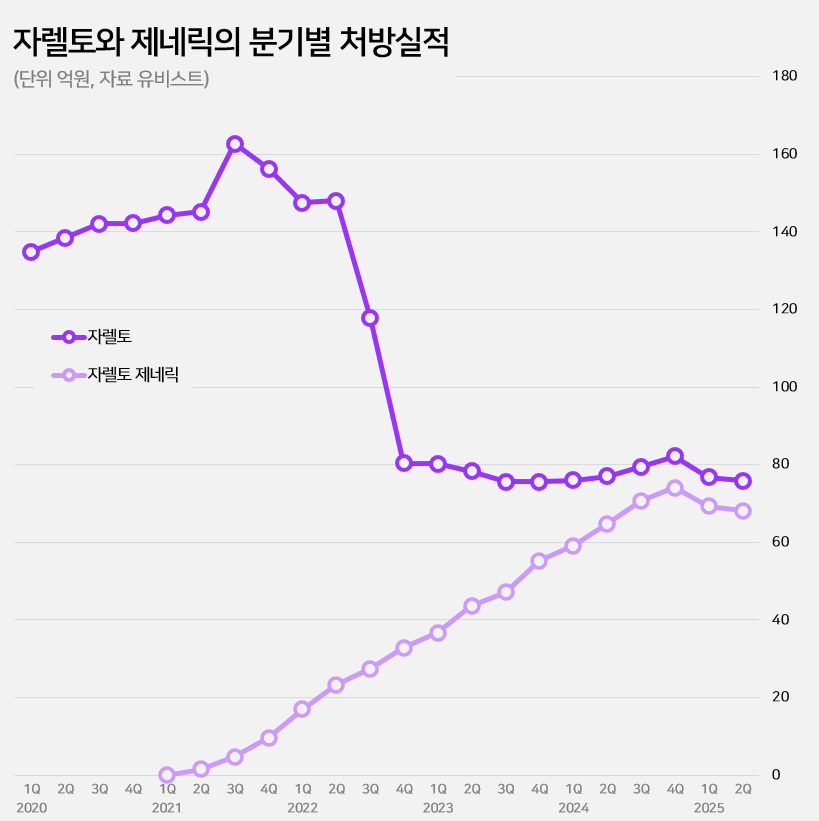
Xarelto recorded sales of KRW 15.3 billion in the first half of this year, maintaining its prescription sales as in the same period last year.
However, the downward trend has been evident since 2021.
Compared to KRW 28.9 billion in the first half of 2021, sales have decreased by half in four years.
This is due to the entry of generic drugs.
Xarelto generics first appeared in the second quarter of 2021.
About 40 products were released simultaneously in line with the expiration of Xarelto’s substance patent.
Subsequently, prescriptions steadily increased, reaching KRW 4 billion in the first half of 2022, KRW 8 billion in the first half of 2023, KRW 12.4 billion in the first half of 2024, and KRW 13.7 billion in the first half of this year.
The remaining products had prescription sales of less than KRW 1 billion in the first half.
As sales of the original product slowed down, generics expanded their influence, and the market share of generics in the rivaroxaban-containing DOAC market reached 47% as of the first half of this year.
Another original product, Pradaxa (dabigatran), is struggling to gain traction in the DOAC market.
Pradaxa's prescription sales in the first half of the year were KRW 4.7 billion, a slight decrease from the KRW 4.8 billion in the same period last year.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










