- LOGIN
- MemberShip
- 2025-12-19 08:06:45
- Fewer drugs reimbursed 5 years into pricing reform
- by Chon, Seung-Hyun | translator Alice Kang | 2025-07-29 06:04:21
Over the past five years, 4,500 drugs have been removed from the reimbursement list in Korea.
The number of new drugs entering the market has decreased significantly with the introduction of generic drug price reforms and joint development regulations.
The number of prescription drug approvals has decreased by more than 80% compared to 5 years ago.
Pharmaceutical companies rushed to enter the generic market ahead of the tighter regulations, and new market entries plummeted after changes were made to the approval and drug pricing systems.
With more products being withdrawn than entering the market, the total number of listed prescription drugs began to decline.
Reimbursed drugs down 17% in 5 years...
Decline continues after drug price reform According to the Health Insurance Review and Assessment Service on the 28th, as of the 1st of this month, a total of 20,277 drugs were listed on the health insurance reimbursement list.
Although this is an increase of 44 from the 21,983 last month, it is a decrease of 1,000 from 23,027 in July last year.
This means that the number of drugs listed on the reimbursement list has decreased by an average of 83.3 per month over the past year.
Approvals for prescription drugs decreased by 84% this year compared to 5 years ago...
Joint development regulations accelerate sharp decline in approvals The number of approvals for prescription drugs has significantly decreased since the reform of the drug pricing system.
As of June this year, the number of approvals for prescription drugs was 315, with a monthly average of 52.5.
Although this is 4.2 drugs more than last year's monthly average of 48.3, it is 23.8 fewer than the 76.3 in 2023, marking a decrease of 23.8 in 2 years.
In the first half of 2020, a total of 2,015 prescription drugs were approved, averaging 335.8 per month.
This is an 84.4% decrease in the monthly average number of prescription drug approvals over 5 years.
The average number of prescription drugs approved per month in 2021 and 2022 was 133.3 and 93.2, respectively, which was significantly higher than the average number approved this year.
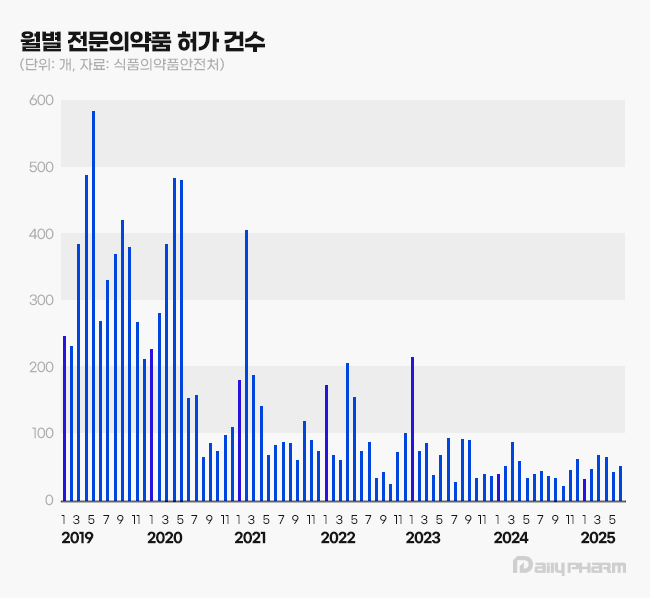
of prescription drugs approved every month (Source: HIRA) The analysis is that the attempts to enter the generic drug market, which accounts for the largest share of new entries in the prescription drug market, have decreased significantly.
The rise in the regulatory barriers for approval significantly dampened the market entry momentum.
Since July 2021, the revised Pharmaceutical Affairs Act has limited the number of incrementally modified and generic drugs that can be approved on a single clinical trial.
The new regulations, known as the “1+3 rule,” restrict the number of incrementally modified drugs and generics that can be approved based on a single clinical trial.
If a pharmaceutical company manufactures its product at the same facility with the same formulation and manufacturing process as the product for which it conducted its own bioequivalence study, the use of that data is limited to three.
In other words, only four generic drugs in total can be approved based on a single bioequivalence study.
Similarly, clinical trial data can be shared with and applied to only 3 other products besides the one directly conducted by the company.
In the past, when one pharmaceutical company obtained approval for a generic drug after bioequivalence testing, dozens of other pharmaceutical companies could obtain approval for their own generic drugs using the same data.
However, due to the joint development regulations, “unrestricted replication of generic drugs” is no longer possible.
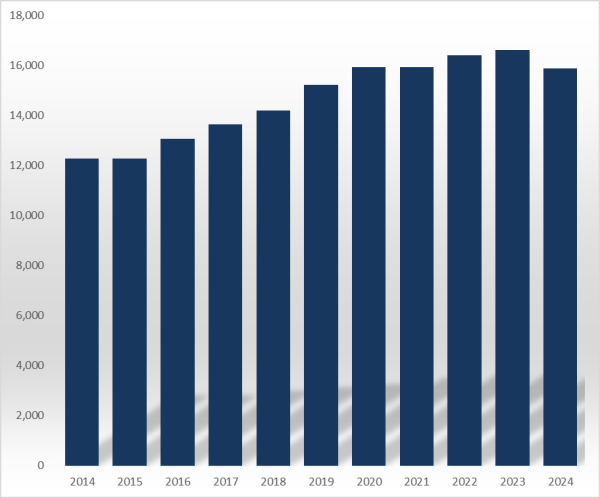
According to the MFDS, the number of prescription drugs approved last year was 15,893, a decrease of 739 from 1,6632 in 2023.
This means that 739 more products had their approvals expire than those approved as prescription drugs in a single year.
The number of prescription drug items had increased every year until 2023, since recording 9,572 in 2010.
During that period, the number of new products entering the market exceeded the number of products withdrawn from the market every year.
However, due to the decrease in prescription drug approvals, an unusual situation occurred last year, with the total number of items decreasing.
The number of prescription drug approvals has increased explosively since 2019, but has turned to a decline since 2020.
In 2018, 1,562 prescription drugs were approved, averaging 130 per month, but in 2019, the number jumped to 4,195, averaging 350 per month, more than double the previous year.
In May 2019 alone, 584 prescription drugs were approved in that single month.
From October 2018 to July 2020, more than 100 prescription drugs were approved each month, but in August 2020, the number of approvals fell below 100 for the first time in 23 months.
Since January 2023, when 216 prescription drugs were approved, the number of prescription drugs approved each month has fallen below 100 for two years and five months.
Companies indiscriminately entered the market in 2019 and 2020 before the introduction of tightened regulations...
Repeated mass withdrawals due to non-production and non-claiming The surge in prescription drug approvals in 2019 and 2020 has been attributed to government policies.
The government's move to tighten regulations on generics led to a surge in generic approvals.
In 2018, 175 items containing the high blood pressure drug valsartan were banned from sale due to excessive impurities.
At that time, the MOHW and MFDS formed a “Generic Drug System Improvement Council” and began to develop measures to curb the proliferation of generics.
When the government signaled its intention to strengthen regulations, pharmaceutical companies moved to secure generic products in advance, leading to a temporary surge in generic drug approvals.
The number of generic drug approvals surged in response to the government's plans to tighten regulations, but returned to previous levels after the system was revised.
At the time, there were numerous cases of pharmaceutical companies withdrawing their generic products from the market without selling them after indiscriminately obtaining approval.
In November last year, over 1,000 drug items were removed from the health insurance reimbursement list due to non-production and non-claims.
Health authorities will remove drugs from the reimbursement list if there have been no insurance reimbursement claims in the last 2 years or no production or import reports in the last 3 years.
This means that 1,000 items were removed from the reimbursement list despite being approved by the Ministry of Food and Drug Safety and being listed for reimbursement because they had no production or sales for a certain period of time.
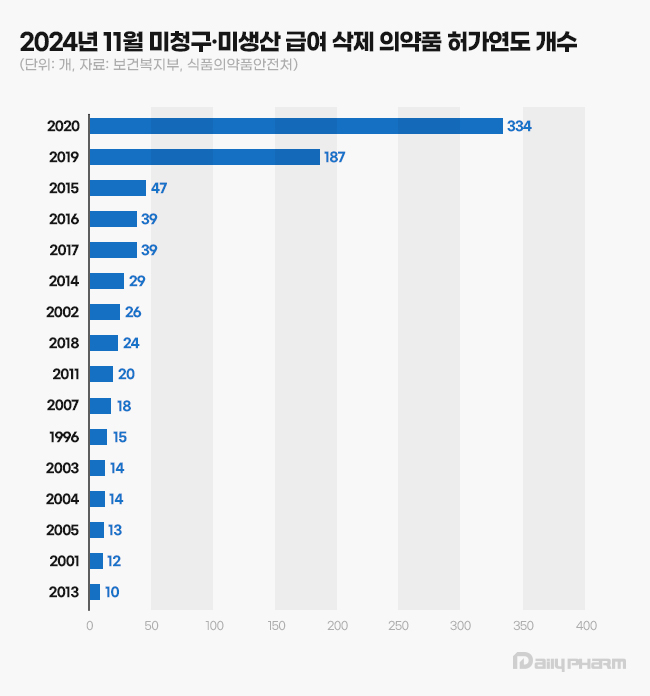
of reimbursement discontinuation drugs due to lack of claims or production by year of approval as of November 2024 (Source: MFDS): Many of the drugs that were removed from the reimbursement list in November last year were approved in 2019 and 2020.
Among the 1,000 drugs removed from the reimbursement list in November last year, 334 were approved in 2000, and 187 in 2019.
Products approved in 2019 and 2020 accounted for more than half of all products removed from the reimbursement list, being 521 products in total.
This means that more than half of the drugs whose reimbursement was revoked were new products that had been on the market for less than 5 years.
Among the drugs whose reimbursement was discontinued due to lack of claims or production, 47 were approved in 2015, and 39 were approved in 2016 and 2017, which was significantly lower than in 2019 and 2020.
Only 24 products approved in 2018 were removed from the reimbursement list, compared to how the number of products withdrawn from the market skyrocketed among drugs approved in 2019 and 2020, Pharmaceutical companies indiscriminately obtained generic approvals in response to the government's strengthened regulatory measures, but many products ended up disappearing from the market without being sold.
The companies pursued an indiscriminate policy of securing as many generic products as possible before the government strengthened its regulations, leading to an unusual phenomenon where products were withdrawn from the market in large numbers after a certain period of time.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










