- LOGIN
- MemberShip
- 2025-12-23 07:24:28
- MNCs ask to “Innovate new drug reimbursement environment"
- by Lee, Jeong-Hwan | translator Alice Kang | 2022-01-27 05:43:51

The policy proposal contained the request to pull forward the timing of insurance coverage for the companies' new drugs with measures such as preparing a separate source of finances other than the NHI finances by setting a new account for pharmaceutical expenses for severe diseases, introducing the ‘pre-(insurance) listing post-evaluation system, diversifying the risk-sharing agreement (RSA) scheme, and introducing a customized reimbursement model, etc.
Also, requests on improving the domestic drug pricing system so that the innovative value of new drugs can be fully reflected in new drugs by improving the pharmacoeconomic evaluation system, and building a control tower for new drugs under the direct control of the president to reinforce nurturing/support/development of innovative new drugs.
The Korean Research-based Pharmaceutical Industry Association will deliver a healthcare policy pledge proposal for the 20th presidential election that contains the contents above to People Power Party Representative Jun-Seok Lee on the morning of the 27th.
The policies KRPIA asked for are laregly: ▲reducing the economic burden of patients by strengthening medical expenditure support ▲ improving the new drug listing system ▲Building a global hub to reinforce capabilities to develop blockbuster new drugs ▲introducing a global level evaluation system to rationalize the drug price decision-making structure ▲establishing a control tower for new drugs under the president.
In other words, KRPIA’s policy proposal implies the need for innovation in NHI listing and reform of Korea's drug pricing system to enhance patient access to new drugs that are owned by the MNCs.
The association saw that the current health insurance finances and catastrophic medical expense support system were insufficient to enhance patient access to new drugs.
As its solution, KRPIA pointed to expanding subjects and the extent of catastrophic medical expense support while preparing a separate source of finances for the reimbursement of severe diseases or high-priced pharmaceuticals.
In particular, the association stressed the need to establish a separate account to cover drug expenses for severe diseases by procuring additional finances from the national treasury and the Health Promotion Fund, as well as with refunds that the NHIS receives from the pharmaceutical company under their RSA contract, and cancer management fund, etc.
The KRPIA also criticized the problems in Korea’s new drug listing system that reduce the use rate of new innovative new drugs compared to other advanced countries while delaying patient administration of such drugs due to the non-existence of an expedited reimbursement evaluation system.
KRPIA suggested that the issue above can be resolved by introducing the ‘pre-listing post-evaluation system that allows for drugs to be listed for reimbursement first then be evaluated by health authorities to decide on a final price, diversifying the risk-sharing agreement (RSA) scheme, and introducing a customized reimbursement model, etc.
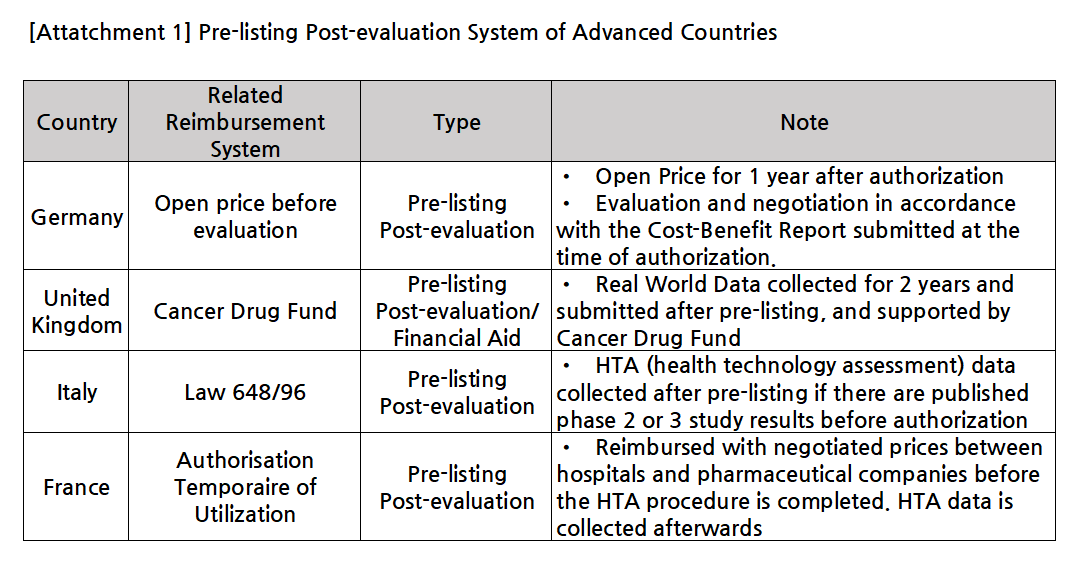
Also, as the current RSA system has a narrow scope of application, KRPIA requested that the RSA scheme cover drugs that have fiscal neutrality, recognized for the therapeutic need diseases other than cancer or rare diseases.
Also, the association suggested introducing a detailed customized reimbursement model that takes into account the characteristics of individual drugs such as advanced biopharmaceuticals, rare disease drugs, and anticancer drugs and preparing an environment in which new drugs can be effectively listed through an expedited reimbursement evaluation system and advance negotiation system, etc.
The KRPIA also raised the need for innovation in Korea’s drug pricing system.
With the claim that the current drug pricing method cannot properly reflect the economic value of many new drugs, the association asked that the innovativeness of a drug be reflected in the drug’s price based on a drug price comparison method.
Also, KRPIA stressed the need to create an environment in which the true value of a new drug is recognized by improving the evaluation process and increasing the transparency and redundancy of evaluations that are undermined during the process of multiple evaluations conducted for new drugs by several committees.
In addition, the association proposed the construction of a control tower for new drugs under the direct control of the president to reinforce the competitiveness of Korea’s pharmaceutical and biopharmaceutical industry and to develop blockbuster new drugs.
New drug support in the current administration is less effective as it is tended to by various ministries including the Ministry of Health and Welfare, the Ministry of Science, Technology, and Information and Communication, and the Ministry of Trade, Industry and Energy, with no control tower to oversee the process.
KRPIA stressed, “We need to open the era of customized health coverage for the people in which the nation covers the innovative treatment of severe rare diseases.
We need to foster Korea to become a blockbuster new drug powerhouse by building an innovative pharmaceutical and biopharmaceutical ecosystem and prepare measures such as introducing an appropriate value appraisal for new drugs.”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










