- LOGIN
- MemberShip
- 2025-12-23 07:21:37
- ‘Lowest-ever’ number of drugs approved in 4 years
- by Lee, Tak-Sun | translator Alice Kang | 2022-01-05 05:59:16
The year 2021 is likely to be remembered as the year of reduced marketing authorizations for drugs.
This was greatly influenced by the changes in the market and the new regulations that were introduced last year.
In particular, the pricing penalty imposed on indirect bioequivalence tested drugs and the restrictions set on consigned bioequivalence tests had greatly contracted the development of consigned generic drugs, significantly decreasing the number of approved items compared to the previous year.
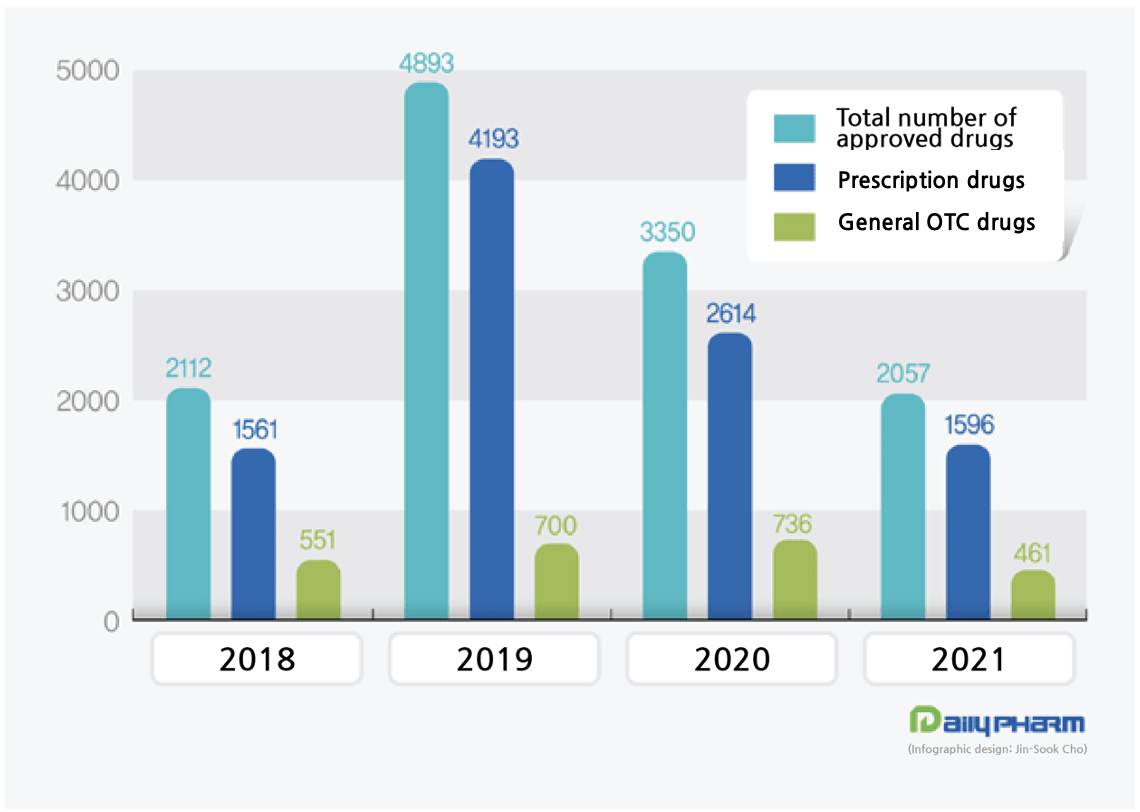
2,112 items were approved in 2018, 4,893 items in 2019, and 3,350 items were approved in 2020.
In the case of prescription drugs, the number of approvals is on a decline for 2 consecutive years since 2019.
After reaching the peak of 4,193 in 2019, the number of approved prescription drugs decreased to 2,614 in 2020 and to 1,596 in 2021.
The situation had worsened significantly due to the introduction of the penalty system that additionally discounts the price of drugs that did not directly conduct bioequivalence tests.
Moreover, the so-called "1+3 restriction” system that was introduced in July regulates the number of pharmaceutical companies that can share a company’s bioequivalence test to three other pharma companies, greatly reducing the foothold of CMO generics.
Therefore, the number of incrementally modified new drugs and generics that conduct their own bioequivalence tests is expected to increase greatly.
OTC drugs are at greater peril.
Compared to the past when OTC development increased when stricter drug pricing regulations were imposed on the industry, the number of approved items decreased to an unusual extent this year.

Since then, the average number of approvals remained in the 100 range, dipping to 88 in September.
◆OTC = Among the 461 OTC drugs that were approved in 2021,6 were data submission drugs, 63 were herbal medicines, and 211 were drugs subject to the Standard Manufacturing Criteria for Drugs, and others including generics accounted for 181.
The drugs ‘subject to the Standard Manufacturing Criteria for Drugs’ that accounted for 46% of the OTC drugs approved this year were allowed sale after simply reporting to the Ministry of Food and Drug Safety.
The MFDS designates the type, standard, and limit of the ingredients, formulation, dosage·regimen, efficacy·effect, and precautions for such drugs so that they may be easily sold.
It is the most preferred type of drug for pharmaceutical companies as it saves development costs.
The reality is that companies cannot invest so much into R&Ds of OTCs that have a small market size and require immense marketing costs.
◆Prescription drugs = Although generic drugs account for a substantial amount of the 1,596 prescription drugs that were approved in 2021, the number of new drugs and data submission drugs are also on the rise.
Among the 45 new drugs that were approved last year, 3 were advanced biological drugs that were newly approved other than reregistered drugs, and 304 were data submission drugs.
These accounted for 22% of all prescription drugs approved in 2021.
The trend was also evident in new drugs approvals.
A record-high number of new active ingredients in 4 years was approved last year.
Many new drugs and vaccines were introduced due to the DOVID-19 pandemic, and 4 homegrown new drugs were also approved last year.
Starting with Yuhan Corp’s anticancer drug ‘Leclaza’ in January, Celltrion’s COVID-19 treatment ‘Regkirona’ in February, Hanmi Pharmaceutical’s neutropenia treatment ‘Rolontis’ in March, and Daewoong Pharmaceutical's ‘Fexclu’ was approved in December.
Also, under the ‘Safety and Support Act for Advanced Regenerative Medicine and Advanced Biopharmaceuticals’ that was implemented in 2020, 3 new advanced biopharmaceutical drugs have entered the market.
These cell therapy and gene therapy products would have been categorized as new drugs in the past.
All three advanced biopharmaceuticals that were newly approved this year are Novartis’ products.
The first advanced biopharmaceuticals ‘Kymriah’ was approved in March, followed by ‘Zolgensma’ in May and ‘Luxturna’ in September.
Novartis’ advanced biopharmaceuticals are all ‘one-shot’ treatments that are leading the pharmaceutical revolution.
However, the drugs have a long way to go before receiving health insurance benefits in Korea as these one-shot treatments for rare diseases are very expensive.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










