- LOGIN
- MemberShip
- 2025-12-23 14:48:52
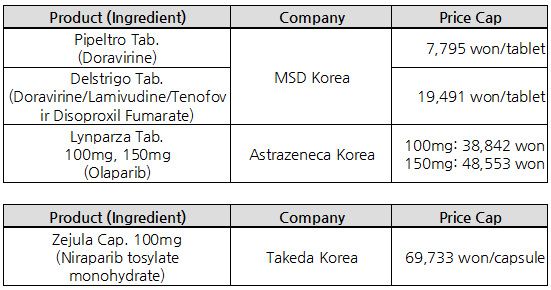
- Delstrigo listed at ₩19,491 … Zejula’s price cut 6%
- by Kim, Jung-Ju | translator Alice Kang | 2021-09-29 05:53:20
MSD Korea’s fixed-dose combination product Delstrigo (doravirine·lamivudine·tenofovir disoproxil fumarate) will be listed with insurance benefit next month, at ₩19,491.
Also, reimbursement for Takeda Korea’s Zejula cap.
100mg (niraparib) has been extended to cover monotherapy for ovarian cancer, upon which the price was cut by 6% and will be reimbursed at the price.
The Ministry of Food and Drug Safety held a Health Insurance Policy Deliberation Committee (HIPDC) meeting today and passed the ‘proposed amendment to the drug benefit list and price ceiling table.’ The amended list and table will be effective from October 1st.
One year after receiving approval from the MFDS on November 22nd, 2019, the company applied for insurance listing of the two products in December last year and received deliberation for the insurance benefit by HIRA’s Pharmaceutical Benefit Assessment Committee in early June.
The alternatives to Pifeltro are the non-nucleoside reverse transcriptase inhibitors Stocrin and Edurant tablets.
And alternatives to Delstrigo are 2 NRTI + NNRTI combo therapies such as Triumeq and ‘Troubadour+stocrin, ‘Descovy+Edurant’.
Pifeltro is listed in all A7 countries abroad, and its weighted average price is ₩24,099 per tablet.
Delstrigo is listed in the U.S, France, Germany, Italy, U.K, Switzerland among the A7 countries, and is its weighted average price is ₩36,483 per tablet.
In the same month, the company had made negotiations with the National Health Insurance Service on the amount of expected claims of each drug.
The NHIS expected no additional funds The final listed insurance price of each drug was set at ₩7,975/ tablet for Pifeltro and ₩19,491/tablet for Delstrigo.
◆Lynparza 100mg and 150mg = AstraZeneca Korea’s Lynparza tab(olaparib) is a treatment for ovarian cancer including fallopian tube cancer or primary peritoneal cancer.
The drug was listed through the risk-sharing agreement scheme (RSA) in October 2017, using the pharmacoevaluation exemption system.
However, its reimbursement was first only allowed for up to 15 months as maintenance therapy after chemotherapy, and then the restriction was lifted in May 2019 after discussions between the government and the company to extend reimbursement.
After then, the company received MFDS approval on October 29th 2019 and applied for insurance benefits in January 2020.
In June of the same year, the agenda was deliberated by HIRA’s Cancer Drug Review Committee, but the company had to reapply for reimbursement in October of the same year.
The drug was finally put on the agenda for deliberation by HIRA’s PBAC in April this year.
In April, the PBAC confirmed that the drug prolonged PFS compared to placebo from a clinical trial.
Also, the committee decided that the ICER is at an acceptable level for use as first-line maintenance therapy and that the drug was cost-effective as second-line maintenance therapy as it cost less than its alternatives, Lynparza cap, and Zejula cap.
The adjusted price of Lynparza tab that is listed in all A7 countries is ₩62,062 for the 100mg tab and ₩67,911 for the 150mg tab.
Based on the calculated price, the company had been negotiating with the NHIS on the drug’s price and expected claims amount.
During the pricing negotiations, both parties agreed on incorporating two types of reimbursement where the company pays back to NHIS a specific portion of the claims amount and a specific portion of the amount of claims that exceed the expected cap.
The final price was set at ₩38,842 for 100mg and ₩48,553 for the 150mg dose.
100mg (niraparib).◆Zejula cap.
100mg = Like Lynparza, Takeda Korea’s Zejula cap 100mg(niraparib) is also a treatment for ovarian cancer including fallopian tube cancer or primary peritoneal cancer that is already receiving reimbursement for the indication.
200mg of the targeted therapy is taken orally twice a day and has been reimbursed as second-line maintenance therapy for ovarian cancer since December 2019, and the reimbursement has been extended to treat patients with recurrent ovarian cancer who have received 3rd-line or higher chemotherapy since February this year.
This time, discussions had been held to extend reimbursement to maintenance monotherapy in ovarian cancer for patients who have responded to first-line platinum-based chemotherapy.
The company succeeded in adding the indication by MFDS on August 3rd last year and has requested an extension of reimbursement in the same month.
In January, the drug has passed CDRC deliberations and was deliberated by the PBAC in June.
At the time, HIRA had determined for reimbursement is appropriate as the drug is recommended as maintenance therapy in ovarian cancer patients who have responded to first-line platinum-based chemotherapy and is cost-effective as it is cheaper than alternative drugs.
Also, it is listed in the U.S, France, Japan, Italy, U.K, Switzerland among A7 countries and its weighted average price is set at ₩129,886 per tablet Since then to earlier this month, the company had negotiated with the NHIS on the drug’s price and expected claims amount.
Both parties had agreed that the company should pay back a specific portion of the amount of claims that exceed the expected cap, and the price to be set at 6% lower than the current cap of ₩74,184 and at ₩69,733.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









