- LOGIN
- MemberShip
- 2025-12-23 14:47:08
- 3 companies voluntarily recall varenicline products
- by Lee, Jeong-Hwan | translator Alice Kang | 2021-09-08 06:05:38

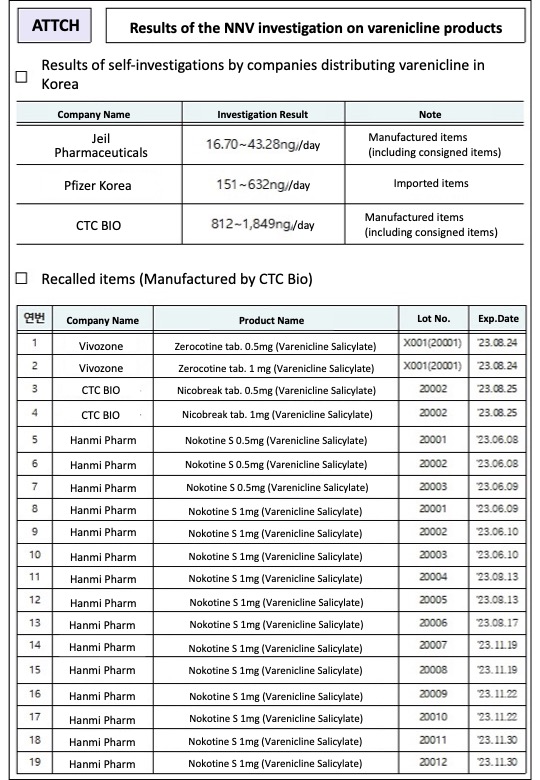
However, all drugs that contain over ‘733ng (nanograms)/day’ of NNV will be voluntarily recalled by the companies in line with the standards set by the U.S regulators, Varenicline products that will be voluntarily recalled include those manufactured (consigned products included) by CTC Bio, and consists of 2 lots of Zerocotine tab.
from Vivozone, 2 lots of Nicobreak tab.
from CTC Bio, and 15 lot of Nokotine S from Hanmi Pharmaceutical.
The MFDS announced the results of the NNV safety investigation on the 7th.
With NNV detected in all products currently distributed in Korea, the Ministry plans to conduct phased safety management measured to assess its effect on administered patients, set standards on the acceptable level of NNV intake, prepare measures for each amount of NNVs detected, and issue guidelines for HCPs and patients.
According to the MFDS, the NNV detected in varenicline products distributed in Korea was very low (16.70~1849ng/day).
More specifically, NNV detected in varenicline products that were sold in Korea were: 16.70~1849ng/day for Jeil Pharmaceuticals’ products, 151~632ng/day for Pfizer Korea’s, and 812~1859ng/day for CTC Bio’s products.
Products from Jeil Pharmaceuticals and CTC Bio were manufactured in Korea (including consigned products), and Pfizer’s was imported.
Assessment of NNV’s effect on the human body also showed a very low level of concern.
The safety investigation results were conducted according to the ICH(International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use) standards, in consideration of the daily maximum dose of intake, NNV test results, the actual period of administration for patients who participated in the NHIS’s smoking cessation treatment support project.
Assessment of NNV’s effect on the human body showed that the NNV brought further cancer risk to 0.194-0.391 out of 100,000 people.
The ICH guideline defines the risk to be at a 'negligible level' if the probability is less than 1 in 100,000 people.
In consideration of the toxicity value set for NTHP, another nitrosamine-class substance with a very similar structure to NNV, the MFDS set the acceptable amount of daily intake of NNV to 37ng/day.
This is the same as the level set by regulation institutions abroad and was determined after consultation with the Central Pharmaceutical Affairs Council.
The acceptable amount of daily intake is the amount that increases the probability of developing cancer by 1/100000 when a substance is taken for a lifetime (70 years), apart from the naturally occurring cancer.
The MFDS decided to allow the lot release of varenicline products that contain NNV of 185ng/day or less for the time being.
This is the same level of standards as the one that was temporarily set by the U.S.
The MFDS decision was affected by the fact that the NNV detected in varenicline was at almost no health risk as found from the NNV human impact assessment, and that it is difficult to immediately reduce the NNV amount to less than the acceptable amount of daily intake (37ng/day).
The MFDS comprehensively reviewed the US's temporary approval of the lot release at 185ng/day, accessibility to smoking cessation drugs for patients, and advice from the Central Pharmaceutical Affairs Council.
In particular, all drugs that are currently in the market whose NNV content exceeds 733ng/day will be voluntarily recalled by the companies.
This is in line with the decision made by the U.S.
to distribute drugs that contain 733ng/day or less without recall.
Accordingly, all lots of the 6 varenicline products from 3 companies that were manufactured by CTC Bio will be voluntarily recalled.
The MFDS plans to continue working with the pharmaceutical industry so that the amount of NNV detected can be reduced below the acceptable amount of daily intake as quickly as possible, and will announce the results after the reductions are complete.
In addition, the MFDS recommended that patients who have already been prescribed the said products (lot numbers) should not arbitrarily discontinue the use of the drug but consult with his/her doctor or pharmacist on continuing taking the drug or switching to a different medication.
If a patient needs to switch to an alternative drug after consultation with his/her doctor or pharmacist, the patient will need to receive a prescription for the alternative smoking cessation treatment from a medical institution participating in the National Health Insurance Service’s smoking cessation treatment support project.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









