- LOGIN
- MemberShip
- 2025-12-21 07:11:06
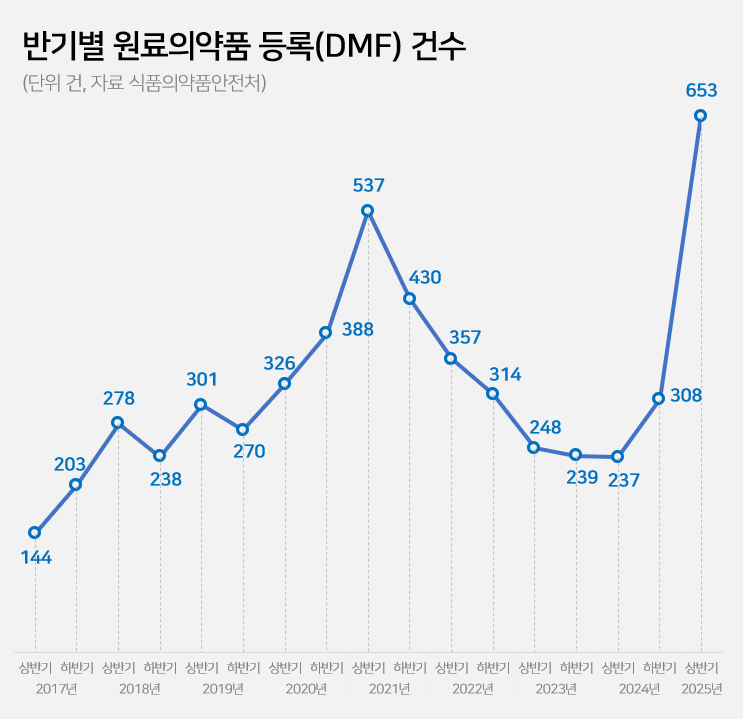
- Drug Master File for API 237→653…'easing of regulation'
- by Kim, Jin-Gu | translator Hong, Ji Yeon | 2025-06-26 06:07:30
The number of Drug Master File, DMF, cases in the first half of this year surged by 2.8 times compared to the same period last year.
This is the highest for a half-year period.
Analysis suggests that this is due to the easing of Active Pharmaceutical Ingredient (API) registration requirements at the beginning of the year.
The government had previously eased regulations to allow GMP evaluation to be replaced by GMP certificates for API registration starting this year.
'Record-High' DMF Registrations of 653 Cases in the first half of 2025...Up 2.8x YoY According to the Ministry of Food and Drug Safety (MFDS) on June 26, the number of API registrations by Korean pharmaceutical and biotech companies in the first half of this year reached 653 cases.
Compared to 256 cases in the first half of last year, this year's marks a 2.8-fold increase in one year.
It has already surpassed the total number of API registrations for the entire 2024 (545 cases).
In terms of half-yearly API registrations, it has exceeded the 537 cases in the first half of 2021, reaching an all-time high.
Earlier this year, MFDS reformed the DMF system to replace on-site inspections with GMP certificates.
Previously, DMF applications required on-site inspections, along with manufacturing facility data, production country manufacturing certificates, and 11 types of GMP documents.
From this year, on-site inspections have been abolished.
Additionally, documents can now be replaced by GMP certificates issued by the government agency of the production country or a PIC/S member country.
The administrative processing period has also been shortened from 120 days to 20 days.
An MFDS official explained, "Previously, to register API, the applying company had to undergo a GMP on-site inspection, but from this year, it can be substituted with a certificate," and added, "It seems that nearly 1,000 piled-up DMF applications were processed in large numbers this year, leading to a surge in DMF cases." Concerns over API quality verification...MFDS states "On-site inspections maintained for high-risk Items" Regarding this deregulation, some in the pharmaceutical industry express concerns that API quality control could become lax.
Critics argue that, with registration now possible solely based on GMP certificates, it will be challenging to identify quality issues beforehand through document-based evaluations.
This easing of regulation is a complete reversal from MFDS's previous stance.
Since the introduction of the DMF system in 2002, MFDS has consistently strengthened quality control.
In 2014, GMP evaluation standards were reinforced with PIC/S membership.
At this time, 11 types of GMP documents and on-site inspection standards were introduced.
In 2019, DMF registration became mandatory not only for new items but also for previously approved items.
In 2021, the on-site inspection system was further strength with a focussing on high-risk items.
During a briefing last year, MFDS explained that they adjusted the evaluation system in response to the administrative bottleneck caused by a surge in DMF applications, which also delayed the review of finished pharmaceutical products (FPP).
Overall, MFDS's policy is to shift its GMP approach to be 'FPP-centric.' Regarding concerns about API quality, MFDS states that on-site inspections are exceptionally maintained for high-risk items, and GMP certificate requirements have been strictly set in line with international standards.
Indeed, for high-risk items such as biopharmaceuticals and sterile APIs, on-site inspections and submission of evaluation data are still required.
Furthermore, on-site inspections are maintained as before for drug approval and suitability judgments.
They also plan to introduce the concept of a 'Site Master File' to understand the quality management system of manufacturing sites comprehensively.
Up and down of cases based on regulatory changes...Decline after 2021 peak→ rebounding This Year The number of DMF registrations by Korean pharmaceutical and bio-companies has fluctuated significantly each year due to system changes and policy factors.
Over the past eight years, DMF cases have exhibited fluctuating trends: ▲347 in 2017 ▲516 in 2018 ▲571 in 2019 ▲714 in 2020 ▲967 in 2021 ▲671 in 2022 ▲487 in 2023 ▲545 in 2024.
With 653 cases in the first half of this year alone, there is a possibility of exceeding 1,000 cases by year-end.
The surge in DMF cases in 2021 coincided with the implementation of a policy that made API registration mandatory, even for previously approved items.
In 2019, MFDS expanded the scope of DMF to include 'previously approved items' from the original 'newly approved items.' It is analyzed that commercial drugs were required to complete registration by 2021, leading to a concentrated influx of DMF applications.
A reform of the drug pricing system around the same time also influenced the increase in DMFs.
In July 2019, the government introduced a 'step-wise drug pricing system.' Generics that did not meet the highest price criteria could maintain their previous drug prices if they submitted data from bioequivalence tests and demonstrated the use of registered APIs.
This led to a surge in DMF applications from pharmaceutical and biotech companies seeking to maintain drug prices.
After 2023, the situation changed.
In February 2023, the submission of DMF documents for drug price maintenance concluded.
With most DMFs for previously approved items also finalized, the number of registrations began to decline.
Indeed, DMFs, which had reached 967 cases in 2021, nearly halved to 487 cases by 2023.
However, with the lowering of DMF hurdles this year, the number of registrations is showing a rebounding trend.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










