- LOGIN
- MemberShip
- 2025-12-17 19:01:30
- NSCLC standard treatment options undergo major change
- by Son, Hyung Min | translator Hong, Ji Yeon | 2025-11-11 06:07:37

With Tagrisso also listed as a preferred therapy in combination with platinum-based chemotherapy, combination therapy is expected to become the major strategy in first-line treatment for EGFR-positive non-small cell lung cancer (NSCLC).
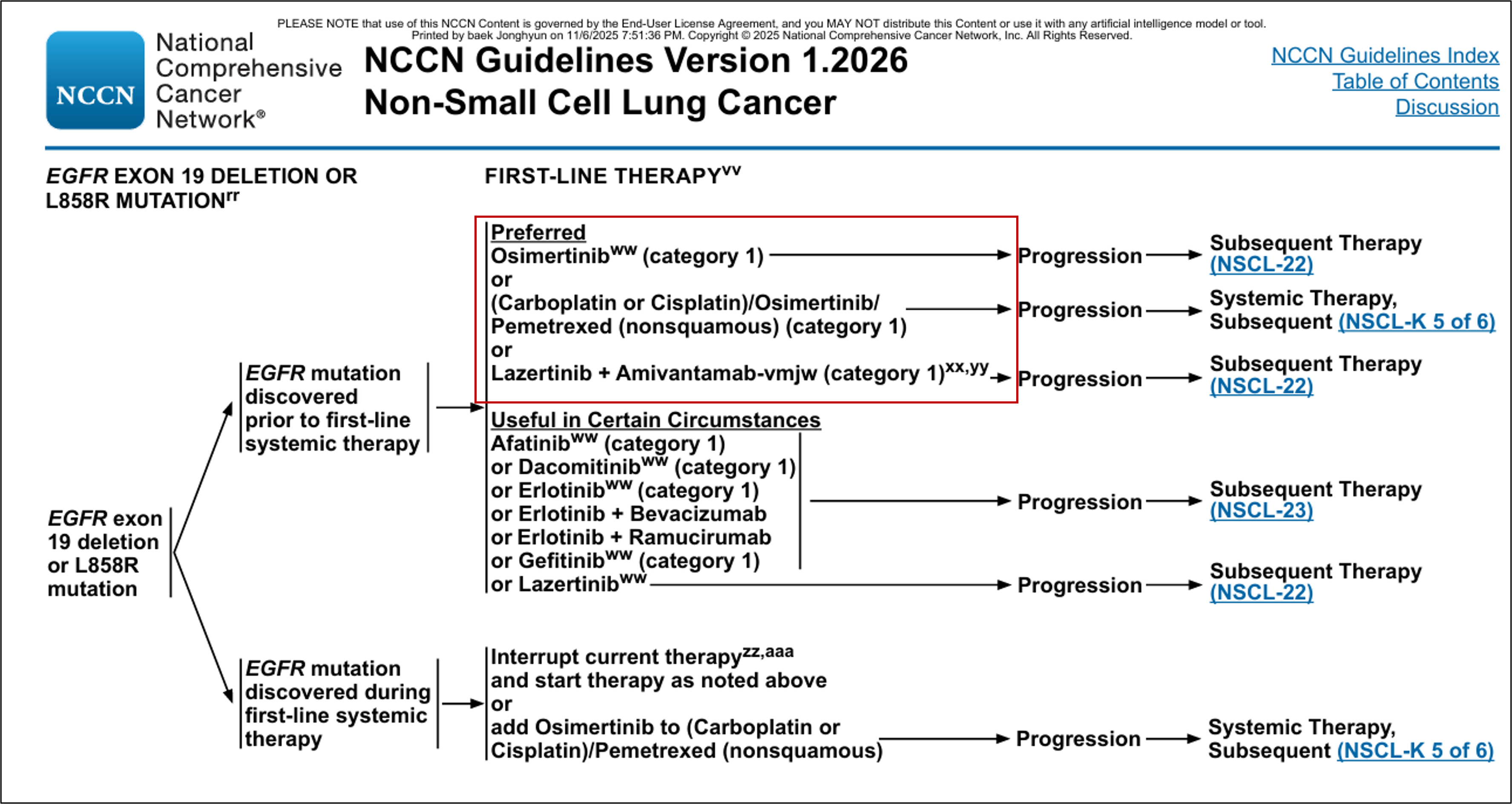
According to industry sources on November 8, the NCCN recently revised its NSCLC treatment guidelines, including the combination of Yuhan's 'Leclaza (lazertinib)' and Janssen's 'Rybrevant (amivantamab)' as a Category 1 Preferred, first-line recommendation for EGFR-positive NSCLC.
Consequently, experts preferentially recommend combination therapy as first-line treatment when an EGFR exon 19 or exon 21 mutation is identified in NSCLC.
The Leclaza + Rybrevant combination therapy, which was first listed as a general recommendation last year, achieved the significant result of being changed to a Preferred recommendation in just about a year.
This opens the door to recognition of combination therapy as a Standard of Care (SOC) globally.
The NCCN provides guidelines for the diagnosis, treatment, and prognosis of cancer.
This organization is a federation of 32 institutions, including U.S.
National Cancer Centers and research institutes, and healthcare professionals worldwide refer to NCCN as a primary guide for cancer care and treatment.
The reason the Leclaza + Rybrevant combination therapy was listed as a Preferred recommendation by the NCCN is that it secured sufficient efficacy results.
Leclaza is a new EGFR-positive NSCLC drug developed by Yuhan, a 3rd-generation tyrosine kinase inhibitor (TKI) targeting exon 19 and exon 21 (L858R) mutations.
Johnson & Johnson secured the global rights to Leclaza and has been conducting clinical research to evaluate its efficacy in combination with Rybrevant, a targeted treatment option that targets exon 20 and the MET mutation.
The final overall survival (OS) analysis of the Leclaza + Rybrevant combination therapy showed superior OS compared to 'Tagrisso (osimertinib)' monotherapy.
This combination therapy is currently expected to secure the longest OS results among all pivotal clinical studies for EGFR-positive targeted therapies.
In the trial, the combination therapy group showed a statistically significant improvement in survival compared with the Tagrisso monotherapy group (p< 0.005).
In detail, the median OS for the Leclaza + Rybrevant group was not estimable (42.9 months-NE).
In contrast, the Tagrisso group showed an OS of 36.7 months.
Based on the distribution of survival indices, the Leclaza + Rybrevant group is expected to extend OS by at least 12 months compared with the Tagrisso group.
These study results were recently published in the New England Journal of Medicine (NEJM), attracting significant attention from the academic community.
Previously, Tagrisso monotherapy, a 3rd-generation TKI targeting EGFR exons 19 or 21, had been recommended as the Preferred treatment option in major guidelines, such as the NCCN guidelines.
However, the Leclaza + Rybrevant combination therapy, which is a 3rd-generation TKI similar to Tagrisso, demonstrated superior results compared to Tagrisso monotherapy, suggesting potential changes in key cancer treatment guidelines.
The Leclaza + Rybrevant combination therapy is achieving significant success in both the OS results, which were identified as the key to commercialization, and its NCCN guideline listing.
Tagrisso + platinum-based chemotherapy receives the priority recommendation…combination with Rybrevant possible
While Tagrisso became the SOC for first-line EGFR-positive NSCLC through monotherapy, the Leclaza + Rybrevant combination therapy joined the competition.
AstraZeneca received approval for Tagrisso in combination with platinum-based chemotherapy as a first-line EGFR-positive treatment.
The company aims to extend survival by using chemotherapy, previously a second-line treatment, at an earlier stage.
The Tagrisso + platinum-based chemotherapy combination therapy demonstrated efficacy compared with Tagrisso monotherapy in the FLAURA2 study.
The clinical results showed that the combination therapy recorded an OS of 47.5 months, longer than Tagrisso monotherapy's 37.6 months.
The median Progression-Free Survival (PFS) based on the investigators' assessment was 25.5 months, an extension of 8.8 months compared with Tagrisso monotherapy at 16.7 months.
AstraZeneca is confirming the possibility of combining its targeted therapies with various options.
The company is currently conducting combination trials of Tagrisso with the Antibody-Drug Conjugate (ADC) 'Datroway (datopotamab)' and the targeted therapy 'savolitinib'.
In addition, AstraZeneca is conducting a clinical trial in collaboration with the global Contract Research Organization (CRO) Parexel to assess the combination potential of Tagrisso and Rybrevant.
The Phase 2 OSTARA study began in July 2023 and is evaluating the efficacy and safety of the Tagrisso + Rybrevant combination therapy as a first-line treatment for EGFR-positive NSCLC.
With the efficacy of the Rybrevant + Leclaza combination therapy validated, AstraZeneca also plans to confirm the potential of combining Tagrisso with a targeted therapy.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










