- LOGIN
- MemberShip
- 2025-12-24 04:52:56
- What are the major systems of the MOHW, the MFDS, & the KCDA
- by Kim, Jung-Ju | translator Choi HeeYoung | 2020-12-29 06:08:54

In addition, the national infrastructure necessary for rapid introduction and verification of the safety and effectiveness of COVID-19 vaccines and treatments using high-tech new technologies will be expanded.
The Ministry of Economy and Finance announced on the morning of the 28th a summary of the government's business plan for 2021, which will have a great impact on the public among all government ministry projects next year.
In the health care and pharmaceutical industry sectors, reinforcement of coverage for rare and essential targets and reduction of entry into essential medical care and advanced and precision technologies are typical.
◆Ministry of Health and Welfare = Diseases subject to the exempted calculation of health insurance will be expanded from January.
The exempted calculation is a type of guarantee that reduces the estimated copayment rate to 10%.
The targets for promotion are patients with severe incurable diseases, rare diseases such as keratoconus patients, or 68 rare diseases such as hydranencephaly and severe atopic dermatitis.
Newly designated as a disease subject to exempted calculation.
For these diseases, the current hospitalization co-pay rate is 20% and the outpatient co-pay rate is 30-60%, and from next month, the inpatient and outpatient co-pay rates will be 10%.
In August 2017, health insurance coverage for chest (breast) and echocardiography will be expanded according to measures to strengthen health insurance coverage.
The MOHW will expand and apply echocardiography in order from the first half of next year to the chest (breast) and from the second half of next year.
Until now, the government has completed health insurance coverage.
Beginning with the upper abdomen in April 2018, ultrasound examinations in the lower abdomen and the urinary system in February 2019, the emergency and critically ill patients in July of the same year, the male genitalia in September of the same year, the female genitalia in February this year, and the ophthalmology sector in September will be expanded.
Health insurance benefit will be expanded if there is a need for examination due to a disease or suspected disease.
The government announced that it plans to discuss specific insurance coverage and medical cost reduction effects, and to prepare related legal procedures later with the medical community.
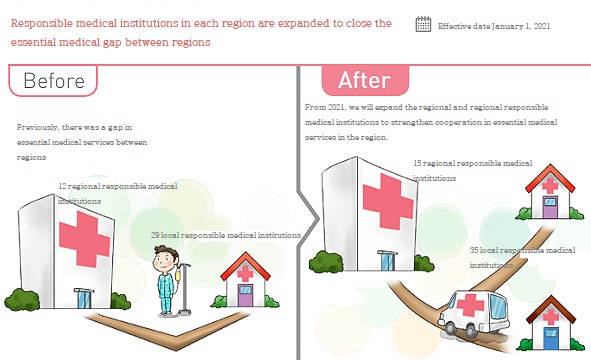
The government has been expanding responsible medical institutions in each region in order to close the gap in essential medical care between regions since last November.
This is a project to strengthen cooperation in essential medical services centered on public hospitals, focusing on 15 regionally responsible medical institutions such as national university hospitals, and 35 regionally responsible medical institutions such as local medical centers.
The main goal is to establish a system of direct and essential medical cooperation.
In addition, the business area in the essential medical field is expanded step by step to develop a cooperation model and carry out the project.
The MOHW is carrying out a project to revitalize medical referrals with the main focus of improving the reimbursement rate system so that exchange of medical information can be activated through an electronic method such as electronic record.
This is a method in which a medical institution in a non-capital region increases the fee when it is requested above the general hospital level (including specialized hospitals) in the same city.
As a result, the government is expecting to establish the function of each type of medical institution and to ease the focus of patients in large hospitals.
In addition, patient treatment and image information will be exchanged through an electronic method so that patient’s referrals can be made.
The government explained that this will increase patient safety by ensuring continuity of treatment at the time of referral and return, and increase patient convenience as patients do not have to submit treatment information directly.
In addition, it added that it is possible to expect efficient use of medical resources and savings in health insurance finances by reducing duplicate examinations and imaging.
In addition, the reimbursement rate system will be improved so that medical institutions in non-metropolitan areas can induce referrals to general hospitals, general hospitals, and specialized hospitals within the same city (17 administrative districts) to strengthen the connection of medical institutions within the local community.
A plan is also underway to shorten the period of entry into the medical field with new medical technologies through regulatory innovation of medical devices.
The MOHW expanded the targets for the evaluation of innovative medical technologies from last November so that cutting-edge medical technologies such as digital therapeutics and precision medicine can enter the medical field early.
Specifically, the number of innovative Health Technology evaluation targets has been expanded from 6 to 9, and the disease group, which was limited to 4 fields, has been abolished, enabling the use of advanced medical technology for various diseases with a large impact on society.
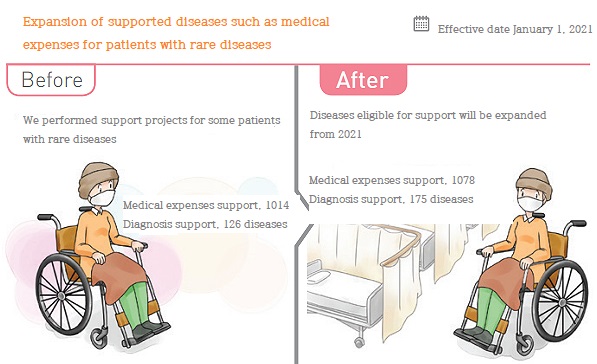
◆The KCDA = The KCDA will expand supportable diseases such as medical expenses for rare diseases from January next year.
The KCDA announced a list of additionally designated rare diseases in October this year, and plans to increase support for rare diseases such as medical expenses support and expansion of target diseases for diagnosis next year.
From January, the number of diseases subject to the medical expense support project for rare diseases has expanded from 1,014 to 1,078, supporting health insurance copayment.
In accordance with the designation of new rare diseases, the number of diseases subject to “genetic diagnosis support for rare diseases” will also be expanded from 126 to 175.
Online applications for medical expenses support for people with rare diseases will also be expanded.
The scope of the online application for the “medical expense support project for people with rare diseases,” which started in March this year, will be expanded from next September.
Specifically, it will expand from low-income health insurance subscribers who do not currently have a support obligation to all low-income household health insurance subscribers, medical benefit patients, and the next higher-ranking out-of-pocket patients who are eligible for deductible deductions regardless of whether they are obligated to support or not.
In addition, from next month, free vaccinations for the elderly pneumococcal (PPSV23) will be expanded from the current public health center to a consigned medical institution.
The government is temporarily expanding operation of vaccination institutions to consigned medical institutions from June 22 to the end of this year in response to COVID-19 by public health centers this year.
If it is expanded to entrusted medical institutions, about 14,000 sites will be added from 256 existing ones.
In addition, the KCDA strengthens the protection of personal information exposure of patients with infectious diseases.
In the event of an infectious disease crisis, personal information such as the name and detailed address of residence that are not related to the prevention of infectious disease should be excluded when disclosing the route of movement of the patient with an infectious disease.
This project will be applied from the 30th of this month.
◆The MFDS = The MFDS will support education and training to nurture experts in regulatory science to strengthen the safety supply of biohealth products and domestic industrial competitiveness from next year.
The government explained that it is essential to develop safety technologies that can evaluate product safety, effectiveness, quality and performance in order for biohealth products to which future technologies (AI, application of big data, etc.) are applied to the market quickly.
Accordingly, the MFDS will select five excellent universities in Korea and provide more than ₩500 million per year to each university for five years in order to cultivate professional researchers who research safety technology while learning legal knowledge about the licensing of biohealth products In addition, universities that have received applications are planning to cultivate more than 600 new researchers and field experts by establishing master's and doctoral degrees in regulatory science in the field of food and medicine at graduate schools.
Product development and safety management for COVID-19 will also be strengthened.
The MFDS is planning to reinforce infrastructure, such as verification of the safety and effectiveness of COVID-19 vaccines and treatments using state-of-the-art new technologies, and national testing equipment necessary for rapid domestic supply.
All vaccines, including the COVID-19 vaccine, must undergo a lot release from the MFDS after reviewing data on manufacturing and quality control and verification tests for domestic supply.
Accordingly, for the rapid and safe national lot release of COVID-19 vaccine, advanced analysis equipment will be added and newly introduced, and a special laboratory will be built.
In addition, in order to accelerate the development of a vaccine and treatment for COVID-19, the country plans to designate an “IRB” for rapid review.
It shortens the entry period for clinical trials by conducting an integrated review (one time) by IRB for approval of clinical trials conducted by various medical institutions.
To this end, the MFDS will regularly review major new information such as side effects of clinical trial drugs to prevent harm to patient safety in advance.
In addition, it will provide a platform that can be used for new drug development by transparently disclosing information from clinical trial approval to results, and release a lot of vaccines quickly and safely to successfully overcome COVID-19.
In addition, a design for the expansion of patient treatment opportunities and the rights, safety and welfare of clinical trial participants will be established.
Mandates education to prevent recidivism in people who commits a crime related to narcotics.
Since the 4th of this month, the MFDS has been convicted by a court or notified of a summary order as a mandatory education to prevent recidivism of people who commits a crime related to narcotics has been implemented.
The crime related to narcotics is a policy that compulsory completion of an order to attend education or rehabilitation programs necessary to prevent recidivism within 200 hours.
In addition, safety inspections for overseas direct purchases will be further expanded.
Even though the rate of nonconformity such as overseas fast-purchased food is very high, there are only about 1600 purchase inspections as of this year, and there is a possibility of domestic inflow of hazardous food, and the government inspects it at twice the level of the previous year (3,000 cases) from next year.
It plans to expand the number of cases.
In particular, the MFDS plans to reinforce the safety management of foods purchased directly from overseas by diversifying inspection targets such as foods of vulnerable groups and foods that are domestic issues from the existing tests focused on improving sexual function, strengthening muscles, and diet food.
The MFDS also implements an innovative medical device software manufacturing company certification system to promote rapid commercialization of innovative medical device software.
Innovative medical device software refers to a software-only product among medical devices with significantly improved safety and effectiveness compared to existing medical devices by applying advanced technologies such as artificial intelligence (AI) and virtual reality (VR).
When manufacturing licenses for innovative medical device software of certified companies, some of the submitted data will be exempted so that innovative products can be quickly released to the market.
The special exceptions for certification and permitting of manufacturing enterprises will be implemented within the first half of next year when the relevant regulations are revised.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









