- LOGIN
- MemberShip
- 2025-12-24 04:53:24
- Secured COVID-19 vaccines for 44 million people
- by Lee, Tak-Sun | translator Choi HeeYoung | 2020-12-10 06:09:19

The remaining doses will be filled with COVAX Facility (about 10 million people) and 4 million Janssen vaccines.
The government announced that it had deliberated and decided on a plan to secure a vaccine developed overseas for COVID-19 and discussed vaccination plans at a cabinet meeting presided over by the Prime Minister on Tuesday.
Through the meeting, the government announced that it will pre-purchase overseas vaccines for up to 44 million people through COVAX Facility (for about 10 million people) and global vaccine companies (for about 34 million people).
With the goal of equally supplying vaccines up to 20% of the population by the end of 2021, COVAX Facility is focused on the World Health Organization (WHO), Coalition for Epidemic Preparedness Innovations (CEPI, vaccine development), and Global Vaccine Alliance (GAVI, vaccine supply).
It is a multinational coalition that is being promoted.
At a meeting of experts in introducing vaccines, it was recommended to secure AZ, Pfizer, Modena, and Janssen vaccines The government has formed Vaccine Introduction Special Team (TF) composed of relevant ministries and private experts from the end of June to secure vaccines quickly, and from July to global companies leading vaccine development such as Pfizer and AstraZeneca.
On September 15th, it was decided to first secure a vaccine that can inoculate 60% of the people (about 30 million people) through participation in COVAX Facility and negotiations with individual companies as a first step through a state council meeting.
It is explained that the government has been reviewing the supply conditions, safety and effectiveness of each company with private experts, and has carefully negotiated to secure a vaccine with good safety, effectiveness and high probability of success.
The government decided to pre-purchase more vaccines than vaccines that can be inoculated by 60% of the population, taking into account the possibility of failure of the developed vaccine after discussing experts in the field of vaccines, and proceeding with the procedure for signing a contract for pre-purchase with global companies.
The committee recommended securing all vaccines from four companies: AstraZeneca, Pfizer, Modena, and Janssen.
Accordingly, the government first purchases up to 64 million doses (for 32 million people) of vaccine through global pharmaceutical companies.
The government pre-purchases 20 million AstraZeneca batches, 20 million Pfizer batches, 4 million Janssen batches, and 20 million batches by pharmaceutical companies.
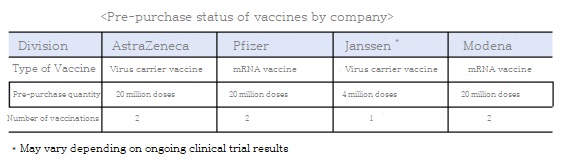
It explained that the pre-purchased vaccine will be introduced in stages from the first quarter of 2021 (February and March), and it will closely monitor the development trend of subsequent vaccine development in the future, and will actively secure additional quantities required.
The government explained that it has secured a budget of about ₩1.3 trillion, including ₩172.3 billion for transfer and exclusive use of the 2020 budget for advance payment of vaccines and purchase of vaccines, for the fourth additional ₩183.9 billion, and ₩900 billion for reserves in 2021.
₩85 billion of ₩172.3 billion is previously executed as an advance payment for joining the COVAX Facility.
Establishment of COVID-19 Vaccination Response Promotion Team, Comprehensive consideration of vaccination timing The government said that it will prepare COVID-19 vaccination system quickly and without disruption.
It was expected that there would be difficulties in the vaccination preparation process due to the storage conditions of the vaccine (Pfizer -70℃±10℃, etc.), short shelf life, two doses and various types.
Regardless of the timing of vaccinations, the government plans to pursue preparations in earnest.
The KDCA is promoting the establishment of a separate organization (COVID-19 Vaccination Response Promotion Team) for vaccine introduction and vaccination.
Vaccine development has not yet been completed with respect to the vaccination timing, and there are still concerns about the safety and effectiveness, so it will be flexibly decided in consideration of ▲COVID-19 domestic situation ▲foreign vaccination trends and side effects ▲national demand.
The government is reviewing the recommended targets for vaccination (approximately 36 million people) for the elderly, the elderly, living in group facilities, chronic diseases, etc.
And it will be reviewed with relevant ministries.
However, the evidence of safety and efficacy for children and adolescents is still insufficient, but the future vaccination strategy will be reviewed through continuous monitoring of clinical trial results.
Minister of Health and Welfare Park Neung-hoo said, “As the vaccine is still in the pre-development stage, and there are still uncertainties about success such as side effects during the vaccination process, we will pre-purchase more than the 30 million people announced by the government for public health and safety.” He said, “As it is expected that the domestic treatment currently being developed will be commercially available as early as next year, a more robust quarantine system can be established with prevention (COVID-19 vaccine)-rapid discovery and diagnosis-early treatment.” He stressed, "As it takes a considerable amount of time to complete the vaccination against the COVID-19 vaccine, the people need to strictly follow the quarantine guidelines, such as distancing in daily life, wearing masks, and refraining from going out."
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









