- LOGIN
- MemberShip
- 2025-12-24 04:54:52
- Reimbursement procedure for RSA drugs has been disclosed
- by Lee, Hye-Kyung | translator Choi HeeYoung | 2020-11-30 06:21:30
The amount of risk-sharing refunds paid to patients by The NHIS for the last five years under the Risk Sharing Agreement (RSA) contract reached ₩5.46 billion.
More than half of them, or ₩3 billion, were paid out from January to September of this year.
Nam-seon Choi, head of the NHIS drug price negotiation department, recently revealed the procedure for paying the patient's co-payment difference at a risk-sharing drug price negotiation system and online information session for follow-up management for more than 100 pharmaceutical companies.
RSA is a system in which pharmaceutical companies partially bear the risks of the efficacy and effects of new drugs or the financial impact of insurance, and pharmaceutical companies must set collateral to the NHIS according to the refund rate.
"After monitoring the RSA drug claim data, the refund amount will be determined and notified according to the contract details with each pharmaceutical company," he said.
"If the pharmacecutical company does not pay to the NHIS within one month, the security right will be exercised after the last notification." When notifying the refund amount = The NHIS monitors the billing data every one year due to the setting of the estimated billing cap for Expenditure Cap among RSA targets.

For example, if a drug is listed in January 2020, after monitoring the treatment from January to December of that year, a notification of the refund amount will be made to the pharmaceutical company around June 2021.
“Other claims are monitored every six months and additional claims are notified.” He said, "In Expenditure Cap, the cap setting is based on the treatment start date, so the monitoring can be notified only when the billing data and the actual payment is accumulated based on the treatment start date." For types other than Expenditure Cap, the amount of refund is notified after 2 months after monitoring of the payments paid every 3 months.
If it is paid from January to March, the notice will be made in May.
He explained, "The NHIS calculates the refund amount for the billed amount, excluding the total amount of copayment, the share of other agencies, and the HIRA's review and adjustments among the requested data for medical institutions." The difference in part of the patient's copayment =How will the actual amount of reimbursement for patients who use RSA drugs will be determined?
In the case of anticancer drugs subject to RSA, the listed price is ₩1 million, and the refund rate is 30%.
Patients receive a copayment of 5% at the time of treatment and pay ₩50,000 as a drug fee.
Choi said, "The NHIS receives a refund of ₩300,000 from a pharmaceutical company, and patients pay ₩35,000, 5% of the actual price of ₩700,000." He also said, "If the refund amount is notified to the pharmaceutical company and the calculation of the refund amount for the previous year's co-payment limit system is completed, based on the completed claim, ₩15,000 will be refunded." However, in order to prevent overlapping support, if the copayment cap refund is larger, the RSA drug refund will not be received.
Patients taking positive listed drugs are paid the difference every quarterly four times a year.
In this way, the NHIS paid the patient's co-payment difference, which increased ₩220 million from 1,020 cases in 2016 and to 1.08 billion, 4,194 cases in 2019.
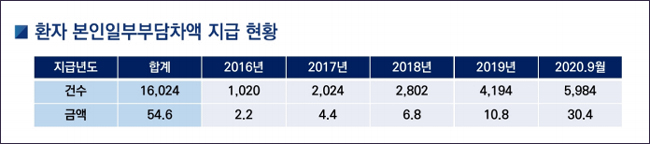
Choi said, "There was an increase in RSA drugs, and as the number of patients with Refund type increased, nearly 6,000 cases were already refunded this year." He added, "It is expected to increase by one thousand by the end of the year." Reimbursement of the difference for patients by themselves in full =In a form in which the pharmaceutical company directly refunds the patient, the pharmaceutical company must notify the NHIS of the payment details monthly.
The NHIS compares the reimbursement details of the pharmaceutical company with the full amount of the claims and sends a notice to the person concerned.
If the medical staff determines and administers it as the subject of full self-payment, it can also be checked with a statement of medical expenses, receipts, and prescriptions as a refund target.
Choi said, "We need to cooperate with pharmaceutical companies so that patients who pay the entire amount will know that the difference will be refunded." "It is easy for patients to receive reimbursement if they need to be in advance.
It is necessary to provide guidance and guides in medical institutions."
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









