- LOGIN
- MemberShip
- 2025-12-22 10:44:24
- Policy
- Australia, excluding drug price reference countries
- by Lee, Tak-Sun Jan 01, 2023 10:40pm
- Australia's addition to the drug price reference country, which faced opposition from the pharmaceutical industry, failed. The HIRA initially decided to take a step back from adding Australia and Canada to the drug price reference country and add only Canada to the reference country. The HIRA released the "Detailed Evaluation Standards for Drugs Subject to Negotiation, including New Drugs" on the 28th on the work portal of nursing institutions. The evaluation criteria include the contents of PE drugs announced by the HIRA in August and the expansion of drug price reference countries announced in November. According to the revised detailed evaluation criteria, Canada was included in the existing countries of Japan, France, Germany, Italy, Switzerland, the United Kingdom, and the U.S. A7 countries, making it A8. Australia, which was announced in November, was not included in the reference country. In the case of Australia, the domestic pharmaceutical industry strongly protested, saying that the price of generic drugs could be lowered if they are used for re-evaluation of post-registration materials due to low generic drugs. The KRPIA also issued a statement of opposition, fearing that the price of new drugs would be lowered. The HIRA includes Canada, which is acceptable to all stakeholders, to secure the validity of the amendment, and Australia is eventually excluded. The HIRA official said, "Australia was added to the drug price reference country due to similar geographical access and economic conditions, but as the pharmaceutical industry's opinion, we decided to exclude it in terms of industrial similarity." If it is possible to omit the submission of PE data, it will be evaluated as the lowest among the national adjustment prices of eight foreign countries of similar drugs when registered in more than three A8 countries. In addition, even if it is difficult or possible to select foreign similar drugs, 10% of the highest price of alternative drugs is added to the list of less than A83 countries, and the adjustment price of countries excluding similar drugs is used for evaluation. The drug A8 is also referred to in RSA drugs. The detailed evaluation criteria also reflected that "a small number of target patients" were among the requirements for the omission of submission of PE data. The number of patients is judged based on the expected number of patients subject to benefits (in Korea) of the indication, but the current status of the expected number of patients at the time of evaluation of the drug, which has been considered essential for treatment, is considered. For drugs that can be used for both adults, the submission of PE data can be omitted only if the main indication is children. This reflects the HIRA's prediction in August. In this regard, there were many objections, saying that the PE exemption system has retreated to limit the number of patients and exempt PE only from pediatric indication treatments. However, the HIRA applied the original plan as it is, saying that these regulations can be flexibly applied in the evaluation process.
- Policy
- New formulations of narcotics will be as strictly reviewed
- by Lee, Jeong-Hwan Dec 30, 2022 06:33am
- Regulations on narcotics for medical use, such as narcotic appetite suppressants and propofol, which is used for general anesthesia, that the government is restricting new approvals for, are expected to become stricter than before. Until now, even narcotic medications for which new approvals are restricted by the government were allowed to receive new approvals when developed into new formulations, but these new formulations will not be approved in the future if they have a high risk of misuse or abuse or is rejected by the deliberation committee. On the 19th, the Ministry of Food and Drug Safety announced that it had publicized the narcotic medications subject to restrictions that contain the restrictions above. More specifically, the MFDS had made the notification on the 28th of last month, upon which the notice immediately took effect. The drugs subject to restrictions are amfepramone and mazindol-containing drugs, GHB and its isomer or drugs that contain its sodium salt, phentermine, phendimetrazine, and propofol-containing drugs. The MFDS had already restricted approvals for the substances on August 14, 2020. One point of attention in the new announcement is that even narcotic medications subject to restrictions that were developed into a ‘new formulation’ may be subject to deliberations by the ‘Narcotics Safety Control Deliberation Committee’ if the MFDS determines the drug to have a high risk of misuse or abuse. Therefore, if the new formulation does not pass the ‘Narcotics Safety Control Deliberation review, that new drug may not receive marketing authorization in Korea. The MFDS announced such restrictions on narcotic medications due to intermittent applications filed for marketing authorizations by companies after changing only the formulation of their drugs to receive approval in categories where new marketing authorizations are restricted. As the authorities restricted new approvals in the above areas to eradicate misuse and abuse of narcotic medications, the MFDS needs to ponder whether to approve the new formulations when a pharmaceutical company requests approvals for developing new formulations. In the case of propofol, a restricted narcotic, the company applied for marketing authorization after changing its injection formulation into a prefilled syringe type. The drug was Fresenius Kabi’s ‘Fresofol MCT Prefilled Syringe.’ As a result, the MFDS issued a public notice and implemented an administrative measure to clearly prohibit approval of new narcotic medications with new formulations, etc., if the drug has a high possibility of misuse or abuse through. Also, by making the drugs subject to the Narcotics Safety Control Deliberation Committee review, the approvals of narcotic medications with new formulations will now be reviewed more professionally and objectively. In other words, narcotic medications that wish to obtain new approvals for their new formulations would need to have little risk of misuse or abuse and pass advisory review from the Narcotics Safety Control Deliberation Committee. An MFDS official said, “Among the narcotic medications that were restricted permission to minimize misuse and abuse, there were cases where companies sought new approvals based on new formulations. We made the announcement to clarify the need to restrict such approvals if their risk of misuse or abuse is high." The official added, “The notice further clarifies our regulations on how narcotic medications, even with new formulations, will not be approved if at high risk of misuse or abuse, or fails to pass deliberations by the Narcotics Safety Control Deliberation Committee.”
- Policy
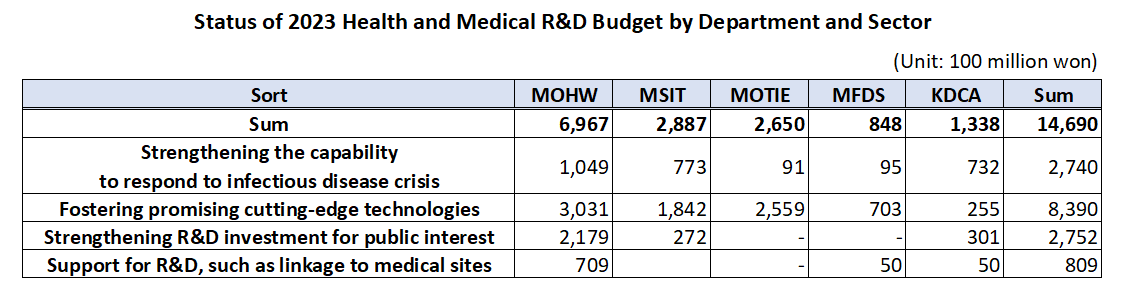
- Gov allocates KRW1.47 trillion budget for healthcare R&D
- by Kim, Jung-Ju Dec 30, 2022 06:33am
- The government’s budget for next year’s healthcare R&D including new drugs, medical devices, and digital transformation to AI-based biohealth is estimated to be around KRW 1.47 trillion. This is the total amount of budget that will be supported by the Ministry of Health and Welfare, Ministry of Science and ICT, Ministry of Trade, Industry, and Energy, Ministry of Food and Drug Safety, and Korea Disease Control and Prevention Agency, an amount similar to this year and 5% of the government’s total R&D budget. According to the MOHW on the 29th, the government plans to support KRW 149.5 billion in new tasks and KRW 1.32 trillion on ongoing tasks, for a total of 128 projects around 4 major areas including ▲reinforcing the ability to respond to infectious diseases, ▲fostering promising advanced technologies in biohealth, ▲increasing R&D investment for public interest including overcoming diseases, etc., and ▲support for R&D linked to the medical field. ◆Reinforcing ability to respond to infectious diseases = The government will be supporting KRW 274 billion for 26 select projects next year to strengthen Korea’s capacity in responding to infectious disease crises. The authorities plan to establish health security by supporting R&D to secure main technology capabilities for vaccines and treatments that can protect public lives and health from future infectious diseases. By ministry, the MOHW will be investing KRW 3.75 billion in developing antiviral treatments in preparation for RNA virus infections (Disease X), and MSIT has allocated KRW 13.3 billion for the Bio & Medical Technology Development Program and the establishment of the National Preclinical Trial Support System. The government also allocated a budget to advance the disease control and prevention system. Based on the lessons learned from the COVID-19 pandemic, the government plans to establish a safe society from new infectious diseases by promoting research on the whole disease control and prevention, etc to advance Korea’s disease control and prevention system. The MOHW allocated KRW 1.6 billion, KDCA KRW 1.3 billion, and MOTIE KRW 0.7 billion for the project to advance the pan-ministerial infectious disease control and prevention system. ◆Fostering promising advanced technologies= The government plans to support a total of KRW 839 billion in 66 projects to foster promising advanced technologies. First, the government will be improving the quality of healthcare by developing data and AI-based technology and prompting the digital transformation of biohealth, and providing personalized healthcare. In terms of new projects that will be initiated by each ministry, MOHW will be investing KRW 6.25 billion in R&D for technology to utilize healthcare MyData and its demonstration project. Also, the MOHW allocated KRW 7.5 billion to the project for developing healthcare technology based on virtual patients and hospitals, and KRW 7.5 billion for the demonstration and introduction of medical institution-based digital healthcare. The authorities will also be investing in the discovery of promising next-generation areas. It plans to continue the search for future drivers of biohealth by supporting R&D in unexplored areas such as microbiome, and in areas that can enhance industry competitiveness by improving the self-sufficiency of core technologies such as advanced medical devices. In terms of new projects that will be initiated by each ministry, the MOHW and KDCA allocated KRW 3.8 billion and KRW 1.3 billion each for the hospital-based human microbiome R&D project. The MFDS has also newly allocated KRW 7.4 billion to support regulatory science for pan-ministerial medical device regulations, and the MOTIE allocated KRW 2.1 billion for the development of interventional medical device technology based on advanced manufacturing technology. The projects also include R&D in regenerative medicine. The government plans to support research to establish a basis for the commercialization of regenerative medicine, such as xenotransplantation R&D projects, to ultimately become a global leader in advanced regenerative medicine technology by securing core and basic source technologies. In terms of new projects that will be initiated by each ministry, the MOHW will invest KRW 6 billion in xenotransplantation R&D projects, the MOTIE will invest KRW 4.5 billion in bio, medical technology development, and stem cell ATLA-based treatment technology for incurable diseases. ◆Strengthening R&D investment for public interest = The government will also allocate KRW 275.2 billion in 28 select projects to invest in R&D for public interest such as overcoming diseases. First, the government plans to ease the socioeconomic burden by focusing on the development of healthcare technologies to overcome diseases that bring high burden to the public such as brain diseases, mental health, and cancer. In terms of new projects that will be initiated by each ministry, the MOHW allocated KRW 4.95 billion in the development of technologies to address issues in the clinical field for diseases related to brain and nervous diseases, KRW 9.63 billion in an R&D project and its demonstration for the development of evidence-based personalized healthcare for cancer survivors, and KRW 0.5 billion for the development of technology for metaverse-based mental health management in the National Center for Mental Health. The government plans to preemptively conduct R&D in healthcare technologies where public demand is expected to surge in line with social changes including aging and the low birth rate, that will contribute to resolving social issues and enhancing the real sense of R&D in public health. For this, the MOHW will support KRW 3.9 billion in R&D of consumer-focused care robots and their service demonstration. ◆ support for R&D linked to the medical field= The government plans to support KRW 80.9 billion in R&D after selecting 8 projects linked with the medical field. First, it plans to reinforce Korea’s global competitiveness by fostering professional manpower that can drive innovation in biohealth, such as expanding research support for new physician-scientists and continuing training for regulatory science experts. For this, MOHW has allocated KRW 4.05 billion for the global research cooperation support project. Seong-ho Eun of the Bureau of Advanced Health Technology Policy MOHW said, “The government will continue to promote relevant policies and continue increasing R&D investments so that the healthcare R&D provided by the government serves as a basis for better quality healthcare services. We will also further activate the Health and Medical Technology Policy Deliberation Committee so that related ministries and the private sector for more organic cooperation and communication.”
- Policy
- A rapid change in the population
- by Kang, Shin-Kook Dec 29, 2022 06:03am
- Policy Tasks Determined at the Second Vice-Minister Meeting of the Ministry Related to Population Future Strategy. The government will come up with all-around measures due to population changes caused by the world's highest rate of low birth rate and aging society. This included institutionalization of non-face-to-face treatment and visiting medical services. On the 28th, the government held the second vice-ministerial meeting of ministries related to population future strategy presided over by Na Kyung-won, vice chairman of the Low Birth Rate and Aging Society Committee, and announced "Demographic Change and Countermeasures." This is because the population decreased by 37.66 million in 2070 due to low birth rates, and side effects such as a surge in the elderly population and the disappearance of the region were imminent. ◆ Non-face-to-face treatment and medical-care supply = In order to improve medical access to books and wallpaper and improve patient health, it will also promote the institutionalization of non-face-to-face treatment centered on primary medical institutions next year. In addition, a clinic-level medical institution that provides visiting medical care and care services will be designated and a pilot project for a home medical center will be implemented this month. In addition, the "contract doctor system" that regularly visits nursing facilities where doctors do not reside to check and manage the health status of patients will be enhanced. ▲ A schedule for promoting the health and welfare sector among major tasks in response to the population crisis The government will also review measures to expand supply by supporting the private sector's entry into the elderly care service sector and inducing diversification and scale. It aims to create a foundation for expanding the private supply of various services by introducing a self-burden system for elderly customized care services and providing universal services. ◆ Regional medical personnel and health insurance financial efficiency = Consultations on adjustment of the number of medical schools to cope with the increase in medical demand due to the expansion of training at local hospitals and aging of majors will also begin. Currently, the medical school has a quota of 3,058. In addition, a pilot project for joint training between national university hospitals and local medical centers will begin in March next year. The government plans to establish a "fiscal vision 2050" in the first half of next year to discover reform tasks for overall economic and social structural problems such as responding to population decline. It supports the discussion of pension reform measures by the National Pension Reform Special Committee and also prepares measures to preemptively streamline health insurance spending. Various pilot projects will be promoted to convert the value-based payment system based on medical service performance, not input, and appropriate medical use inducement measures will be prepared, such as rationalizing the use of non-benefits and linking public health insurance. Disclosure of non-benefits, non-benefit reporting by medical institutions, and establishment of a health insurance-loss insurance-related management system are expected to be on the agenda. "The world's fastest-growing demographic change will have a wide impact on the economy and society, including education, military service, local economy, growth potential, industrial structure, and welfare system," a government official said. "In the short term, the growth potential is weakened due to the aging population."
- Policy
- Takeda CMV infection treatment Livtencity has been approved
- by Kim, Jung-Ju Dec 29, 2022 06:03am
- After transplantation of Takeda Pharmaceutical Korea, Cytomegalovirus infection treatment Livtencity obtained domestic item permission and passed the first gateway to supply. The Ministry of Food and Drug Safety (Director Oh Yoo-kyung) announced on the 27th that it has approved Livtencity of Takeda, a rare drug. Cytomegalovirus (CMV) is asymptomatic and is reactivated, causing serious diseases. Livticity is an oral antiviral drug that inhibits the growth of the virus by lowering the activity of the "UL97 protein phosphorylation enzyme" involved in cloning and proliferation in CMV. The drug is expected to provide new treatment opportunities for adult patients with macrophage virus infection after transplantation, which is resistant to or unresponsive to one or more of the existing antiviral drugs Ganciclovir, Valganciclovir, Foscavir, and Cidofovir.
- Policy
- Abbott’s Lipidil NT 145mg listed with reimbursement
- by Lee, Tak-Sun Dec 27, 2022 06:10am
- The original developer of the hyperlipidemia treatment fenofibrate will be releasing a 145mg product that can be taken on an empty stomach. This is the second drug to be released, following Yuhan Corp. As such, the two products are expected to compete fiercely in the market next year. According to industry sources on the 26th, Abbott Korea’s Lipidil NT (fenofibrate 145mg) will be listed at a ceiling price of KRW 339 from the first of next month. The price has been set the same as Yuhan Corp’s Fenowell Tab 145mg. The Health Insurance Review and Assessment Service decided for Lipidil NT’s price to be set at the same level as Yuhan Corp.'s Fenowell Tab 145mg was the only identical drug available and its price had already been adjusted to 53.55% of the original’s price. Although Lipidil NT had been listed later than Yuhan’s drug, its entry is expected to receive attention for being produced by Abbott, the original fenofibrate drug developer. Fenofibrate 160mg tablets are currently mainstream in the market, and the drugs recorded KRW 165 billion in outpatient prescriptions (UBIST) last year. The current market leader, GC Corp’s ‘Lipidil Supra,’ is also a 160mg strength tablet. Lipidil Supra has been developed by Abbott. However, the 160mg tablets have the inconvenience of needing to be taken with the main meal due to absorption issues in the stomach. On the other hand, gastrointestinal absorption of the 145mg tablets are absorbed quicker, and therefore can be taken with or without meals. As the new strength supplemented the shortcomings of existing products, there have been prospects that prescriptions will shift to the 145mg formulation with their release. Due to this element, a fierce competition is expected in the market with Yuhan Corp when Abbott releases Lipidil NT Tab The industry believes the result between the two companies will depend on how well Yuhan Corp preoccupied the market for the 5 months since its reimbursement listing in July this year. However still, it is analyzed that Abbott may quickly absorb the market as the original maker, even though it is the latecomer in the market. If GC Corp, which has already been targeting the domestic market in partnership with Abbott, adds on its support, Lipidol NT’s penetration rate in the market may accelerate further. GC Corp has also been approved for a 145mg product. Its product is Neofeno Tab. The product is being manufactured by Yuhan Corp upon consignment, but unlike Yuhan Corp, the company has not applied for reimbursement. Accordingly, there has also been analysis that GC Corp will collaborate with Abbott in marketing Lipidil NT, just like for Lipidil Supra.
- Policy
- Darzalex succeeded in renewing his contract with RSA
- by Kim, Jung-Ju Dec 26, 2022 06:07am
- Darzalex, a treatment for multiple myeloma by Janssen Korea, has succeeded in negotiating a contract renewal with the NHIS. As a condition for renewal of the contract, the drug price was reduced by 2% by content, and RSA plans such as refund rate and cap were also set. According to the industry, the Ministry of Health and Welfare will push for a revision of the "drug benefit list and upper limit table," which includes Darzalex price cut, based on the renewal of the RSA contract between the NHIS and the company. This drug is used to treat multiple myeloma, which failed to treat at least three treatments, including proteasome inhibitors and immunomodulatory agents, and the alternative drug is Dexamethasone which is listed in all of the A77 countries (the United States, France, Germany, Italy, Britain, Switzerland, and Japan). When the RSA-applied drug approaches the expiration of the contract period, the NHIS evaluates the clinical usefulness and cost-effectiveness of the drug in advance. Based on the results, the corporation and pharmaceutical companies renegotiate RSA negotiations on the reduction price, expected claim amount, refund rate, and cap. The drug succeeded in signing its first RSA contract with the NHIS on April 8, 2019, as a Refund with Expenditure Cap, and was set to expire in early April next year. The company conveyed its intention to renew the contract to the NHIS, and renegotiated with the NHIS from September 23 to the 24th of last month after the NHIS review. As a result, the drug price by content was reduced by 2%. The NHIS expected to cut the drug price to 2% compared to the existing upper limit, which is expected to reduce the budget for health insurance. The time to apply the cut price is April 8th next year.
- Policy
- Dong-A ST’s Forxiga prodrug Dapapro 5mg listed for reimb
- by Lee, Tak-Sun Dec 26, 2022 06:07am
- Following the reimbursement listing of the 10mg formulation of Dapapro Tab, the first follow-on drug of the antidiabetic SGLT-2 inhibitor Forxiga that had been developed by Dong-A ST, the 5mg lower-strength formulation of the drug is also soon to be listed with reimbursement. When listed, Dapapro Tab 5mg will be the only dapagliflozin drug to be reimbursed and will have the potential to exclusively take over the market. Reimbursement of the original Forxiga 5mg has been removed from the list. According to industry sources on the 23rd, Dong-A ST’s Dapapro Tab 5mg will be listed at an insurance ceiling price of KRW 456 per tablet. Dong-A ST had already launched Dapapro Tab 10mg with reimbursement for KRW 684 per tablet starting this month. This was the first Forxiga latecomer to be listed for reimbursement. Other Forxiga latecomers can only be released after the original drug’s patent expires in April. Therefore, the company is expected to make every effort to preoccupy the market before the entry of its competitors. Dong-A ST was able to release its product before patent expiry because its product is a prodrug that has a different chemical structure from the original. However, once absorbed, the structure changes and the drug shows the same effect as the original. Dong-A ST was able to invalidate 917 days added to Forxiga’s substance patent through its prodrug strategy, with the court ruling in favor of Dong-A ST in the passive trial to confirm the scope of the patent that was filed against Forxiga’s substance patent. However, the final winner of the patent suit is yet to be determined as there is a high possibility that AstraZeneca, the patent holder, will appeal. However still, DongA-ST decided to release Dapapro Tab 10mg to preoccupy the market. The Dapapro Tab 5mg that will soon be listed will also be released to the market. Moreover, DongA-ST’s Dapapro Tab 5mg is the only 5mg formulation to be reimbursed. The original Forxiga 5mg Tab had been removed from the reimbursement list in October 2018. As the 5mg strength is the recommended initial dose for patients who have not been treated with drugs for diabetes before in combination with metformin, it is expected to be quite well used in the market. However, AstraZeneca is focusing on the main dose, 10mg strength of dapagliflozin. This is why its combination drugs containing 5 mg of dapagliflozin, Xigduo XR Tab 5/500mg, and Xigduo XR Tab 5/1000mg still remain reimbursed. Therefore, Dong-A ST will be on its own in the dapagliflozin 5mg market until AstraZeneca’s patent expires in April next year. However, dozens of dapagliflozin follow-on drugs are expected to pour in after patent expiry. In addition, Daewoong Pharmaceutical is also aiming for an early release of its SGLT-2 class new drug 'Envlo Tab.’ Therefore, competition among domestic companies in the SGLT-2 inhibitor market will intensify after April. Therefore, from Dong-A ST’s perspective, the next four months will serve as an important period for them to measure the drug’s performance.
- Policy
- Zavicefta has been approved for domestic use
- by Lee, Hye-Kyung Dec 26, 2022 06:06am
- .The Ministry of Food and Drug Safety (Director Oh Yu-kyung) announced on the 22nd that it has approved Korea Pfizer Pharmaceutical's "Zavicefta 2g/0.5g (Ceftazidime/Avibactam)," a new antibiotic drug. This drug is a combination of Cefalosporin-based antibiotic Ceftazidime and avibactam, a newly developed beta-lactam inhibitor. Permitted efficacy and effect are ▲Infection treatment in the complex abdominal cavity for adults and children over 3 months old ▲Infection treatment in the complex urinary tract including nephritis for adults and children over 3 months old ▲Infection treatment in hospitals for adult patients over 18 years old. It is a drug developed by Zavicefta AstraZeneca and sold the development and copyright of the global market outside the U.S. in the low-molecular antibiotic business to Pfizer on August 24, 2016. The Ministry of Food and Drug Safety said, "We will continue to do our best to expand treatment opportunities to patients by quickly supplying treatments that have been sufficiently confirmed in safety and effectiveness based on regulatory science expertise."
- Policy
- Exclude Australia from the drug price reference country?
- by Lee, Tak-Sun Dec 26, 2022 06:06am
- Insurance authorities, which are seeking to expand drug reference countries from seven to nine from next year, are expected to exclude Australia from strong opposition from the pharmaceutical industry. It is said that the government has turned to a careful review after a meeting with the Vice Minister of Health and Welfare and the pharmaceutical industry held last week. The HIRA is expected to finalize the plan announced by internal regulations within this week and announce it soon. According to industries on the 22nd, there is a possibility that an amendment excluding Australia will be announced in the revised bill, which was announced by adding Australia and Canada to the drug price reference country. Earlier, the HIRA announced on the 21st of last month a revision to regulations on evaluation standards and procedures, including whether drugs are subject to concessionary benefits, including the U.S., Britain, Germany, France, Italy, Switzerland, and Australia. It will take effect on 1 January next year. The HIRA explained the revision, "We are using the adjusted price, which translates the drug price of seven foreign countries (A7), to evaluate the adequacy of new drug benefits, but we want to improve transparency and clarity and supplement its validity." When the revision was announced, the pharmaceutical industry strongly protested. In addition to the KPBMA, which represents domestic pharmaceutical companies, KRPIA, which has multinational pharmaceutical companies, issued a statement and made clear its opposition to the addition of Australia. Domestic pharmaceutical companies are concerned that the addition of Australia, which has low generic drug prices, could significantly lower the domestic generic drug price in the re-evaluation of registered drugs that reflect this. Foreign pharmaceutical companies are also strongly opposed to the revision that if Australia, which has low drug prices, is added, the price of new drugs will be lowered, further reducing patient accessibility. At a meeting with Park Min-soo, the second vice minister of the Ministry of Health and Welfare, pharmaceutical organizations, and CEOs of pharmaceutical companies on the 16th, the industry expressed concern about Australia's addition to the reference country. At the meeting, Vice Minister Park also said, "We will listen to the opinions of the field under the stance of increasing the sustainability of the health insurance finance and carefully look at ways to improve the system, including insurance drug price policies." It seems to have changed to a careful review. For now, the Ministry of Health and Welfare is said to have presented its revised opinion, and it is believed that it felt burdened to announce the existing plan as it is, conscious of the controversy within the HIRA. A senior HIRA official also said, "As the purpose of the amendment was to supplement the validity of the existing calculation formula, we are carefully considering ways that all stakeholders can accept." The HIRA is expected to draw up a final draft as early as this week and immediately announce the amendment without further notice.