- LOGIN
- MemberShip
- 2025-12-18 16:38:11
- Policy
- Industry requests incentives for rare and orphan drug supply
- by Lee, Hye-Kyung Aug 29, 2025 06:07am
- The pharmaceutical industry has voiced the need for incentives to expand supply support, such as official certification, for companies supplying rare and essential drugs. The Ministry of Food and Drug Safety (MFDS, Minister Yu-kyoung Oh) visited the Korea Orphan & Essential Drug Center on the 28th to review the center's current projects supporting the supply of rare and essential medicines. It then held an ‘On-site discussion forum to support drug supply’ with the Korean Organization for Rare Diseases, the Korean Society of Health-System Pharmacists, the Korean Society of Cardiology, Huons, Korea United Pharm, and Sinwon Chemical. Pharmaceutical companies attending the meeting reportedly proposed measures such as incentive schemes and ways to shorten the emergency introduction period for rare and essential medicines. Patient groups requested guidance on the supply plans for medicines that had recently experienced supply disruptions, resulting in treatment difficulties. MFDS Minister Yu-Kyoung Oh said, “We will actively support and continuously communicate with the industry and patient groups to ensure the stable and prompt supply of rare and essential medicines needed for treating patients with rare and intractable diseases.” Young-Rim Kim, Director of the Korea Orphan & Essential Drug Center, said, “We will work with the MFDS to reflect the opinions provided by patient groups and pharmaceutical companies in the operation of the rare and essential medicines supply support project and ensure these medicines are safely supplied to patients.” The Ministry of Food and Drug Safety (MFDS), together with the Korea Orphan & Essential Drug Center, is working to supply rare disease medicines in a timely manner and reduce patient burden by rapidly introducing orphan drugs and operating patient support programs. The center is also pursuing self-sufficiency and reducing reliance on foreign countries by utilizing the technological development of raw and finished drugs for essential national medicines that are taken daily through consignment production with domestic pharmaceutical companies. The MFDS plans to continue communicating with patient groups and industry stakeholders to identify and implement various policies ensuring the stable supply of medicines in need. Meanwhile, this forum was arranged to thoroughly explore government support measures for the stable supply of medicines to patients with rare and intractable diseases, an issue raised during the ‘Food and Drug Policy Connection Open Forum (Medical Products Sector)’ held on July 22. Opinions presented at the meeting will be actively reflected in future policies for the stable supply of medicines.
- Policy
- Next year’s reimb reevaluations to be discussed further
- by Lee, Tak-Sun Aug 29, 2025 06:07am
- The government has decided to continue discussions on the 2026 reimbursement adequacy reevaluation plan. While the reevaluation was initially expected to be approved this month through the Health Insurance Policy Deliberation Committee review, it is reported that disagreements remain unresolved regarding selection criteria, procedural improvements, and the ingredients targeted for 2026. Some suggest the findings from an ongoing post-listing drug control study commissioned by the MOHW may be incorporated. According to industry sources on the 28th, the ‘Plan to Promote Drug Reimbursement Adequacy Reevaluations’ reported to the HIPDC subcommittee was not placed on the agenda for the HIPDC plenary session on the 28th. The government stated it would continue discussions. The plan reported to the subcommittee shows that the selection criteria for evaluation will change from the current threshold of an increase of 0.1% or more of the average claim amount over three years (approximately KRW 20 billion) to claims of KRW 10 billion or more. Furthermore, the condition for “'listed reference countries' will be expanded from the current requirement of fewer than 2 countries to fewer than 3 countries. The seven drug ingredients subject to reevaluation in 2026 are: Ginkgo biloba extract that was approved in 1989, Calcium dobesilate hydrate, Calladinogenase, Meglumine gadoterate, Diacerein, Afloqualone, and Octilonium bromide. The level of reimbursement will also be differentiated based on evaluation results. For drugs with unclear clinical utility but high social necessity, a 50% selective reimbursement rate will apply; for those with low social necessity, an 80% selective reimbursement rate will apply. Additionally, if the drug cost is high compared to its alternatives, an additional price cut will be applied. Previously, decisions only excluded drugs from reimbursement or maintained reimbursement, with drug prices adjusted through voluntary reductions by manufacturers to demonstrate cost-effectiveness. The pharmaceutical industry has clearly stated its opposition to this plan. Specifically, the argument is that the late selection of next year's reevaluation targets has rendered insufficient time to prepare data, making the 2026 reevaluation unfeasible. This is because it takes about a year for drug efficacy to be included in textbooks. Additionally, opinions have emerged that the selection of ginkgo biloba extract preparations as next year's target ingredients is unreasonable. So the pharmaceutical industry has expressed temporary relief as the government decided to further discuss the reevaluation plan. It is known that the government authorities were not in agreement regarding this reevaluation plan at the Drug Reimbursement Evaluation Committee and the Health Insurance Review and Assessment Service subcommittee level. Particularly, it is reported that further discussion would be needed regarding changes to the criteria. Some predict that the reimbursement reevaluation plan will be revisited based on the ‘Study on Integrated Post-listing Control of Drug Prices’ currently underway as an MOHW research project. This study is scheduled to be conducted by the Catholic University of Daegu Industry-Academic Cooperation Foundation (Director Hyeop-sang Yoon) until November. It is anticipated that the study conducted last year by the Korea Institute for Health and Social Affairs will include implementation plans related to integrated post-listing control measures. An industry official explained, “Given that the post-listing control study is currently underway, it appears the new administration will comprehensively review the post-listing control system. Consequently, there are projections that the finalization of the reevaluation plan may extend beyond this year.”
- Policy
- Bill banning reverse payment agreements passes committee
- by Lee, Jeong-Hwan Aug 28, 2025 06:10am
- The amendment to the National Health Insurance Act, which regulates "reverse payment agreements" where original drug companies and generic drug companies collude by exchanging money to delay or not release generics, thereby avoiding a price reduction for the original drug, passed the National Assembly Health and Welfare Committee review on the morning of the 27th. The illegal collusion among pharmaceutical companies to withhold generic drug launches has forced patients to pay unfairly high prices for medications. This practice must be eradicated to eliminate such disadvantages and prevent unnecessary leakage of health insurance funds. The bill on revising the Pharmaceutical Affairs Act, which expands the post-notification system for generic substitution at pharmacies to the information system established and operated by the Ministry of Health and Welfare and the Health Insurance Review and Assessment Service, and improves the definition of essential medicines and expands support to include drugs with no substitutes or facing supply instability, has also cleared the Health and Welfare Committee. These bills will take effect according to the implementation date specified in the supplementary provisions once they pass the plenary session after review by the Legislation and Judiciary Committee and are promulgated by the government. Original-Generic Pharma Company Collusion Prohibition…Price cuts or reimbursement suspensions applied for offenses The core provision of the National Health Insurance Act amendment bill proposed by Democratic Party of Korea lawmaker Young-seok Seo stipulates that if the Fair Trade Commission uncovers collusion where a generic drug company agrees not to launch a generic in exchange for exclusive domestic distribution rights from an original drug company, the price of the unfairly traded drug will be reduced or its reimbursement suspended. The bill passed by the Health and Welfare Committee amends Article 41-2 (Reduction of Upper Limit Amount for Reimbursement Costs for Drugs, etc.) of the National Health Insurance Act. Specifically, the bill stipulates that for cases violating Article 40(1) or Article 45(1) of the Monopoly Regulation and Fair Trade Act, where the violation was committed “for the purpose of increasing or maintaining the reimbursement price ceiling for drugs,” the insurance price of the drug can be reduced or reimbursement suspended. The amendment allows for a maximum 20% reduction in drug prices when a reverse payment agreement violation is first detected. If another reverse payment agreement is confirmed within 5 years of the price reduction, the price can be cut by up to 40%. If a second violation of the reverse payment agreement is detected within five years after the second price reduction, the application for reimbursement for the drug can be suspended for up to one year. The effective date for the provisions preventing reverse payment agreements is ‘the day six months after the government's promulgation’. Consequently, collusive reverse payment agreements will now face the same level of penalties as illegal pharmaceutical rebates, including drug price cuts and reimbursement suspensions. The Health and Welfare Committee explained, “This bill enables the reduction of the maximum reimbursement amount for drugs related to unfair joint conduct or unfair trade practices, and the suspension of reimbursement coverage. It will prevent pharmaceutical companies and others from profiting through unfair joint conduct or unfair trade practices and establish a fair drug sales order.” Post generic substitution notification system expanded to HIRA A revision to the Pharmaceutical Affairs Act, proposed by Democratic Party of Korea lawmakers Young-seok Seo, Su-jin Lee, and Byung-duk Min, was passed as an alternative bill by the Health and Welfare Committee. It expands the pharmacists’ post-dispensing notification system to the information system operated by the Health Insurance Review and Assessment Service (HIRA). The bill incorporates the existing regulation requiring pharmacists to inform patients when substituting a drug listed on a prescription with an item recognized by the Ministry of Food and Drug Safety as bioequivalent (generic substitution) and to notify the prescribing physician or dentist within one day (or three days if unavoidable circumstances exist). The key provision approved by the Health and Welfare Committee is the establishment of a new Article 27-2 (Establishment and Operation of a Substitution Dispensing Information System) in the Pharmaceutical Affairs Act, enabling the Minister of Health and Welfare to establish and operate an information system to support post-notification of generic substitution.. Notably, the bill allows the Minister of Health and Welfare to delegate this task to the Health Insurance Review and Assessment Service (HIRA), with the necessary details to be stipulated under the Ministry of Health and Welfare ordinance. The effective date for the simplified generic substitution regulations is set for five months after the government's promulgation. This establishes the legal basis for the Ministry of Health and Welfare and HIRA to build and operate a generic substitution information system to support post-notification of generic substitution under the Pharmaceutical Affairs Act. It is expected that this will enable more efficient communication of generic substitutions while clarifying the accuracy of such notifications, thereby enhancing information sharing between physicians and pharmacists. Expansion of the Definition of Essential Medicines & Legal Basis for the National Essential Medicines Stable Supply Council The Committee also passed a revision to the Pharmaceutical Affairs Act that improves and expands the definition of essential medicines to include those without substitutes or facing supply instability, and elevates the legal basis for the National Essential Medicines Stable Supply Council from a presidential decree to a law. The bill provides a detailed definition of essential medicines. These include medicines essential for maintaining the national health system, such as disease control and radiation disaster prevention (Item a), and medicines that are essential for healthcare but lack substitutes or face supply instability despite market mechanisms (Item b). Furthermore, a new Article 83-5 (National Essential Medicines Stable Supply Council) was added to the Pharmaceutical Affairs Act. This mandates the establishment of a National Essential Medicines Stable Supply Council within the Ministry of Food and Drug Safety (MFDS) to develop countermeasures for both national essential medicines and medicines that, while not designated as such, require stable supply due to temporary increases in demand. Notably, the composition of the National Essential Medicines Supply Council, previously chaired solely by the MFDS Vice Minister, was expanded to include ‘one senior official from the Ministry of Health and Welfare designated by the Minister of Health and Welfare,’ bringing the total to two chairs. This appears aimed at strengthening national management capabilities by adding the Ministry of Health and Welfare to the MFDS as a government ministry with authority over national essential medicines. Furthermore, the legal basis for the composition of the council members was clarified. The effective date of the bill was set as one year after its promulgation. While the definition of essential medicines is being expanded, the government's basis for supporting medicines without substitutes and medicines with unstable supply requiring stable supply due to temporary increases in demand is expected to be strengthened.
- Policy
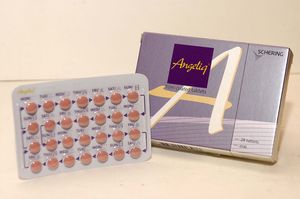
- Reimb listing approved for the first generic of 'Angeliq'
- by Lee, Tak-Sun Aug 27, 2025 06:07am
- Bayer The first generic of Angeliq Tab (drospirenone·estradiol, Bayer), a hormone-based medicine for use in postmenopausal women, will be included in the reimbursement listing. Analysis suggests that the commercialization of domestically produced generic is significant, considering that there had been supply issues related to Angeliq, which is an imported medicine. According to industry sources on August 26, Dalim BioTech's 'Anzeno Tab' will be added to the reimbursement list on September 1 with a ceiling price of KRW 5,565. The product is manufactured directly by Dalim BioTech. Anzeno Tab is a generic drug with the same active ingredients as Bayer's Angeliq Tab. Angeliq is approved for ▲Hormone replacement therapy for estrogen deficiency in women who are at least one year post-menopause, and for the ▲Prevention of osteoporosis in post-menopausal women who are intolerant of or have contraindications to other approved drugs and have an increased risk of fracture. Based on 2024 UBIST data, Angeliq's outpatient prescription sales amounted to KRW 12 billion. Because it is a hormonal drug requiring a separate manufacturing facility had previously prevented generic products from entering the market. Consequently, when supply issues arose with the imported original drug, Angeliq, pharmacies had no identical alternatives, leading to significant difficulties. Alternative prescriptions with similar drugs were the only option, resulting in a continuous demand for generic development. In 2021, the persistent shortage of Angeliq due to production delays at Bayer's Berlin plant caused difficulties for pharmacists. With the launch of this in-house-manufactured generic, the issue of supply instability is expected to be largely resolved. The price of Anzeno Tab was set at KRW 5,565, which is 53.55% of Angeliq's price, without any additional premium, as it met all the required criteria. Angeliq Tab's current ceiling price is KRW 10,393.
- Policy
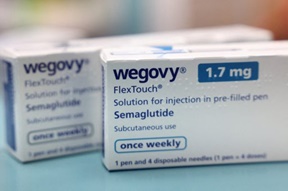
- ‘80,000 Wegovy prescriptions issued per month’
- by Lee, Jeong-Hwan Aug 27, 2025 06:06am
- The popular obesity drug Wegovy, which was launched in Korea in October last year, has already been prescribed about 400,000 times within eight months, raising concerns over potential misuse and abuse. On the 25th, Rep. Kim Sun-min of the Rebuilding Korea Party disclosed the ‘Annual and Monthly Status of Wegovy Prescriptions based on DUR’ data submitted by the Health Insurance Review and Assessment Service (HIRA) and urged safe usage. According to the data, in 2023, the first month of release, the number of prescriptions flagged by the Drug Utilization Review (DUR) system for Wegovy was 11,368. This increased to 16,990 prescriptions the following month, November. The number then reached 21,457 prescriptions in December. This year, the numbers have increased by over 10,000 cases every month to record 22,051 in January, 31,512 in February, and 47,597 in March. Prescriptions then surged to 70,666 in April, then 88,895 in May. In June, the number slightly decreased to 84,848. This means that since its launch in October last year, Wegovy prescriptions averaged 43,931 per month, rising to an average of 57,594 per month this year. Saxenda prescriptions also showed a similar pattern. The number of Saxenda prescriptions in Korea, which was launched in 2018, was 138,353 in 2022, 171,230 in 2023, and 205,109 last year. Experts have pointed out that while these figures alone cannot determine the exact number of recipients—as one patient may have received multiple prescriptions—the steep increase in numbers clearly indicates a rising trend in the number of people receiving injections. Rep. Sun-min Kim stated, “The number of prescriptions via DUR data is exceeding 80,000 per month. Given the recent craze for obesity treatments, we expect significantly more people are actually receiving prescriptions.” Rep. Kim emphasized, “Given the frequent reports of adverse events, obesity treatments must be used safely under the thorough care of specialists.”
- Policy
- MFDS will intensively monitor Wegovy and other obesity drugs
- by Lee, Hye-Kyung Aug 26, 2025 06:05am
- The Ministry of Food and Drug Safety (MFDS) has designated ‘Wegovy’ and other GLP-1-class obesity injectables as targets for intensive monitoring and plans to continuously monitor side effects in collaboration with the Korea Institute of Drug Safety & Risk Management. Th MFDS designates the intensive monitoring status to drugs with high safety concerns in Korea and abroad, or those of high social interest due to concerns about misuse, and intensively collects, analyzes, and reevaluates their adverse events. On the 25th, the MFDS emphasized that injectable GLP-1 class obesity drugs, which are currently attracting significant public attention, should only be used under the supervision of medical professionals for patients diagnosed with obesity, and only in accordance with the approved indication per prescription. Injectable GLP-1 class obesity drugs are prescription drugs indicated for adult obese patients with an initial body mass index (BMI)* of 30 kg/m2 or higher, or adult overweight patients with a BMI of 27 kg/m2 or higher but less than 30 kg/m2 and one or more weight-related comorbidities such as hypertension. According to clinical trial results, even when used within the approved indications, gastrointestinal adverse reactions (nausea, vomiting, diarrhea, constipation, etc.) and injection site reactions (rash, pain, swelling, etc.) are common, and serious adverse effects such as hypersensitivity reactions, hypoglycemia, acute pancreatitis, cholelithiasis, and fluid loss may occur. Also, some medications are contraindicated in patients with underlying conditions such as thyroid medullary cancer, so consultation with a healthcare professional is mandatory. In patients with type 2 diabetes, hypoglycemia and retinopathy may occur, so caution is particularly advised when administering such drugs to patients with relevant medical history. Obesity treatments are prescription-only drugs that must be used under a doctor's prescription with the guidance of a pharmacist. The MFDS urged the public not to purchase or distribute these medications through online platforms, overseas direct purchases, or personal sales. Additionally, the Ministry of Food and Drug Safety recently urged companies holding the marketing authorization rights for the relevant products to prevent misuse and prohibit false advertising, and informed medical professionals about the proper use of obesity treatment medications within their approved scope. The MFDS plans to publish an informational brochure (leaflet) in collaboration with the Korea Institute of Drug Safety & Risk Management, outlining the conditions for which obesity treatment drugs are used, proper administration methods, storage and disposal instructions, precautions during administration, and procedures for reporting adverse reactions (side effects). The MFDS stated, "We will continue to strengthen the provision of information to improve users' understanding of obesity treatment drugs and ensure their safe use."
- Policy
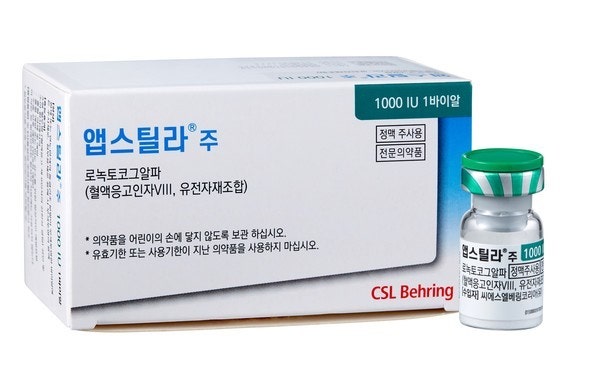
- SK chemicals' Afstyla will be removed from the reimb list
- by Lee, Tak-Sun Aug 25, 2025 06:08am
- Product photo of Afstyla SK chemicals' hemophilia A treatment, Afstyla, which was out-licensed by the company, will be removed from the reimbursement list due to a lack of record of supply performance. The product's delisting was previously deferred in 2023 through conditional negotiation, but with no sales to show since, the decision to remove it has become final. This is a regrettable outcome for a product developed by a domestic company and out-licensed globally. According to industry sources, Afstyla will be removed from the reimbursement list, effective September 1. The product received a decision for removal in 2023 after a review of non-billed drugs revealed no sales over the previous three years; however, its delisting was put on hold through conditional negotiation. However, with no sales recorded within the negotiation period, the decision to remove it has been finalized. Afstyla is currently on the reimbursement list with a price cap of KRW 625 per dose. Afstyla is a biosimilar developed by SK chemicals and out-licensed to CSL Behring Australia during the clinical trial phase in 2009. CSL Behring completed the trials and received approval for the drug in the U.S. and Europe. In 2020, it obtained domestic approval and was added to the reimbursement list in 2021. SK Plasma, an affiliate of SK chemicals, was responsible for domestic sales. In 2020, it obtained domestic approval in Korea and was added to the reimbursement list in 2021. SK Plasma, an affiliate of SK chemicals, was responsible for domestic sales. However, entering the hemophilia market in Korea proved difficult due to the strong presence of established pharmaceutical companies, such as Green Cross, which has a solid position with the hemophilia market. It is also believed that the market outlook was not bright due to the successive launch of competing new drugs. In a 2023 review of non-billed drugs, the product had no production track record for the past three years. However, the manufacturer was able to delay delisting by explaining that sales would soon occur or production would be completed. But after two years with no sales, Afstyla will be removed from the reimbursement list. CSL Behring launched Idelvion, a hemophilia B treatment with improved patient convenience, in Korea last year. With the delisting of Afstyla, it appears CSL Behring will shift its focus to supplying Idelvion.
- Policy
- NHIU ‘Eradicate rebates with INN-based prescriptions'
- by Lee, Tak-Sun Aug 25, 2025 06:06am
- The National Health Insurance Trade Union stressed the need to eradicate pharmaceutical rebates by improving the drug pricing system and distribution structure to those of advanced country standards. The union also advocated the introduction of generic substitution through international nonproprietary name prescriptions. On the 18th, it was revealed through a prosecution investigation that a pharmaceutical wholesaler had provided approximately KRW 50 billion in illegal rebates to three general hospitals through a new scheme involving the establishment of a shell company to pay dividends. The NHIU stated, “The inflated drug costs resulting from illegal rebates and bid-rigging are being passed on to the public in the form of higher medical expenses, leading to unnecessary overprescription of medications and causing serious harm to the health and lives of citizens. This serves as a major factor contributing to the leakage of Koera’s health insurance finances, ultimately resulting in increased health insurance premiums for both citizens and businesses.” The provision of rebates by medical professionals is strictly prohibited under the current Medical Services Act (Article 23-5) and the Pharmaceutical Affairs Act (Article 47). Despite this, in July, the Korea National Police Agency's National Investigation Headquarters announced that it had identified 597 individuals in the medical and pharmaceutical fields suspected of illegal rebates through a special crackdown on illegal rebates and public official corruption. This investigation result demonstrated that illegal rebates in the medical field remain widespread despite the introduction of the dual punishment system for rebates 15 years ago. According to the NHIU, recent illegal rebates in the medical field have been cleverly evading enforcement by exploiting investigative blind spots caused by limited manpower in law enforcement agencies such as the police and prosecution, through more indirect and sophisticated methods such as academic support and consulting. In some cases, an “indirect supplier” is involved as an additional distribution channel between wholesalers and medical institutions, and to circumvent the principle of “one person, one facility” under the Medical Services Act (prohibiting duplicate establishments), wholsalers have been registering indirect suppliers under the names of family members or others related to the medical professionals, leveraging their monopolistic position to extort unfairly low prices from pharmaceutical suppliers and provide illegal rebates to medical professionals. Recently, the Organization for Economic Cooperation and Development (OECD) reported in its ‘Health Statistics 2025’ that South Korea's pharmaceutical expenses are 47% higher than the average of OECD member countries. The NHIU stated, “Enforcement and punishment alone are insufficient to break the vicious cycle of illegal pharmaceutical rebates that recur every year. Fundamental solutions include improving drug pricing systems and distribution structures through price competition among suppliers, such as government tenders and individual drug price negotiations, or introducing flexible pricing systems like reference pricing. Also, the activation of generic substitution based on product names or ingredient names, which is implemented or recommended practice in most advanced countries with separation of prescribing and dispensing systems, is necessary.” They cited Spain as an example. Spain, which has a similar population to South Korea, saves EUR 200 million annually (as of 2017, with a generic substitution rate of 53%) through generic substitution using the International Nonproprietary Name (INN). The union stated that introducing generic substitution through INN-based prescriptions in South Korea could result in annual savings of at least KRW 500 billion in health insurance funds. The NHIU declared, “To eliminate pharmaceutical rebates that threaten the health insurance budget, we will form a united front with labor and civil society organizations that share our vision and set up a proactive and systematic management strategy to improve the drug pricing system and pharmaceutical distribution structure.” It added, “The government and National Assembly must actively pursue legal and institutional reforms to eradicate illegal pharmaceutical rebates. This is because the ‘black money’ from illegal pharmaceutical rebates should no longer be passed on to the public, who already pay the world's highest pharmaceutical costs.” The statement emphasized that pharmaceutical rebates, which exacerbate the public's medical expenses and hinder the sustainable development of the health insurance system, must be eradicated through reforms to establish a drug pricing system and distribution structure on par with those of advanced countries.
- Policy
- IV formulation of Korea’s first IL-23 drug Tremfya approved
- by Lee, Hye-Kyung Aug 25, 2025 06:05am
- The intravenous injection formulation of Tremfya, the first interleukin-23 (IL-23) inhibitor in Korea, was recently granted approval, paving the way for the supply of all Tremfya injection formulations in Korea. On the 21st, the Ministry of Food and Drug Safety approved Janssen Korea's Tremfya Intravenous Injection (guselkumab, recombinant DNA). In addition to receiving approval for ‘Tremfya Prefilled Syringe’ in 2018 and ‘Tremfya OnePress Autoinjector’ in 2021, the company has now received approval for the intravenous injection formulation and may now introduce the formulation to Korea. The indications for Tremfya vary depending on the formulation. The pre-filled syringe formulation is indicated for plaque psoriasis, palmoplantar pustulosis, psoriatic arthritis, ulcerative colitis, and Crohn's disease. The intravenous injection recently approved is used for the induction therapy of ulcerative colitis and Crohn's disease. The 200mg intravenous injection is administered intravenously over a minimum of 1 hour on weeks 0, 4, and 8, followed by maintenance therapy using Tremfya Prefilled Syringe Injection or Tremfya OnePress Autoinjector. Therefore, the intravenous injection is indicated only for the treatment of moderate-to-severe active ulcerative colitis or Crohn's disease in adults who have not responded adequately, have lost response, or have intolerance to conventional therapies, including biological agents or small-molecule drugs. Meanwhile, Tremfya is the first and only interleukin-23 inhibitor approved in Korea for psoriatic arthritis, and its reimbursement criteria are being continuously expanded. Psoriatic arthritis is a chronic progressive immune disease characterized by joint inflammation, enthesitis (inflammation at the sites where bones, tendons, and ligaments meet), dactylitis (severe inflammation of the fingers and toes), and pain in the hands and feet, commonly occurring in individuals aged 30–50 years. To date, there is no cure for psoriatic arthritis, and despite available treatment options, many patients experience symptoms that impair their ability to perform daily activities. It is estimated that approximately 9% of psoriasis patients in South Korea develop psoriatic arthritis.
- Policy
- Blockbuster drugs, Atozet·Rosuzet, get price cuts
- by Lee, Tak-Sun Aug 22, 2025 06:07am
- It has been reported that prices for blockbuster drugs, including the hyperlipidemia combination therapies Atozet (Organon) and Rosuzet (Hanmi Pharmaceutical), are expected to be reduced due to increased usage. These hyperlipidemia combination therapies are frequently subject to annual volume-based drug price negotiations (PVA). Additionally, Celltrion's antibody biosimilar Remsima and Dong-A ST's growth hormone product Growtropin-II Inj are also reported to be on the list for price cuts. On August 21, industry sources reported that reimbursement caps for several products would be adjusted as of September 1, under the 'Type-Da' Price-Volume Agreement. The number of blockbuster products is reported to be included this time. The reimbursement cap for Organon's hyperlipidemia combination therapy, Atozet Tab (atorvastatin-ezetimibe), is expected to be reduced by 3.4% following negotiations. The prices of the 10/10mg, 10/20 mg, 10/40 mg, and 10/80 mg products will be adjusted as follows: 10/10 mg from KRW 951 to KRW 918, 10/20mg from KRW 1,209 to KRW 1,168, 10/40mg from KRW 1,299 to KRW 1,255, and 10/80mg from KRW 1,387 to KRW 1,340. Atozet's outpatient prescription sales last year, based on UBIST data, were KRW 118.7 billion, a 16.3% increase from KRW 102.1 billion in the previous year. The increase in prescription sales alone exceeded KRW 16 billion. This year's negotiation targets for Type-Da Price-Volume Agreement include products whose 2024 claim amount increased by more than 60% compared to 2023, or products that increased by more than 10% with the increase exceeding KRW 5 billion. Based on UBIST data, Atozet meets these criteria. Hanmi Pharmaceutical's hyperlipidemia combination therapy, Rosuzet Tab (rosuvastatin-ezetimibe), is also reported to have undergone a price cut. Rosuzet Tab's reimbursement cap is expected to be reduced by 1.3% to 2.1% depending on the dosage. The prices for the 10/10mg dose are expected to be reduced from KRW 1,103 to KRW 1,087, the 10/20mg dose from KRW 1,111 to KRW 1,093, the 10/5mg dose from KRW 789 to KRW 779, and the 10/2.5mg dose from KRW 727 to KRW 712. Rosuzet recorded outpatient prescription sales of KRW 210.2 billion last year, a 17.6% increase from KRW 178.8 billion in the previous year. HK inno.N's Rovazet and Yuhan Corp.'s Rosuvamibe, which contain the same ingredients as Rosuzet, will also have their reimbursement caps lowered in this negotiation. Rovazet recorded outpatient prescription sales of KRW 47.4 billion last year (up 23%), and Rosuvamibe recorded KRW 89.1 billion (up 14.6%) based on UBIST data. Celltrion's antibody biosimilar , Remsima, used for the treatment of rheumatoid arthritis and other conditions, will also have its price adjusted due to increased usage. Remsima is a biosimilar of Remicade, a blockbuster product with global sales of over KRW 1 trillion. Its reimbursement cap is scheduled to be lowered by 1.2% through an agreement with the NHIS. Dong-A ST's growth hormone product, Growtropin-II Inj, is also on the rise.Growtropin-II Inj's reimbursement cap was adjusted last year due to the Price-Volume Agreement as well. Additionally, Janssen's ADHD treatment Concerta OROS ER Tab is expected to see a 3.9% reduction in its reimbursement cap. Meanwhile, this year's Price-Volume Agreement price reduction list reportedly does not include any of the choline alfoscerate products for cognitive enhancement. These products, which included six items last year, had been consistently on the PVA list. However, after the decision was made to apply selective reimbursement to choline alfoscerate products in a reimbursement re-evaluation, pharmaceutical companies shifted their business to alternative products, which seems to have halted their growth.