- LOGIN
- MemberShip
- 2025-12-22 20:55:25
- Policy
- Ceprotin, Takeda's rare dz, gives 11 yrs for reexamination
- by Lee, Hye-Kyung Aug 12, 2022 05:57am
- On the 2nd, Takeda's Ceprotin, which was first approved as a treatment for severe congenital protein C deficiency in Korea, was given an 11-year review period after marketing. Severe congenital protein C deficiency is a rare genetic disease that causes fatal defects in blood clot control due to a lack of protein C, a type of vitamin K-dependent anticoagulant, and is defined when protein C levels are less than 1% (less than 1 IU/dL), and the incidence rate is estimated to be 1 in 4 million newborns. Ceprotin is the first human protein C drug approved for patients with severe congenital C protein deficiency and was approved for "prevention and treatment of venous thrombosis and fulminant purple hemiplegia in patients with severe congenital protein C deficiency in children and adults." Prior to Ceprotin's approval, the Central Pharmaceutical Review Committee's Pharmaceutical Affairs Subcommittee-Rare Medicine Subcommittee discussed the validity of the re-examination period when licensing biopharmaceutical items. On this day, the committee members decided that it was appropriate to set a period under the Rare Disease Control Act in consideration of the prevalence rate as an item prescribed for rare diseases. It also said that 11 years of re-examination, including pediatric indicators, should be given to secure sufficient safety information. It is reasonable to grant a 10-year re-examination period, including a pediatric indication, and a 10-year re-examination period, which was consulted by the central pharmacy, considering the pharmacological law, the re-examination system, and the characteristics of rare diseases. "It is difficult to judge that there is no appropriate treatment for severe congenital protein C deficiency because there are standard treatments," a member said. "However, it is confirmed that there are limitations on standard treatments and risks to be applied to children." The opinion is that it is difficult to evaluate post-marketing safety due to a lack of patients by granting a 4-6 year review period for congenital genetic diseases, which have symptoms mainly in the early stages of newborns and have a very high mortality rate from complications. Considering the nature of special diseases and the characteristics of the domestic medical environment, a total of 11 years of re-examination period, including pediatric diseases, will be given.
- Policy
- Gov is reluctant to demand that infants be exempted from PE
- by Lee, Jeong-Hwan Aug 11, 2022 06:03am
- The government expressed reluctance to apply PE omission to rare drugs for infants, children, and adolescents. They say that it is a matter to be carefully considered in consideration of other drugs. On the 10th, the MOHW responded to a written question by Choi Hye-young of the Democratic Party of Korea, a member of the National Assembly's Health and Welfare Committee. Choi asked if there is a plan to expand rare drugs for infants, children, and adolescents to PE omission even if they are not treated for rare diseases. The Ministry of Health and Welfare expressed its position that it is difficult to exempt PE from rare drugs for infants, children, and adolescents, along with fundamental answers, such as explaining the criteria for omission of PE data. The Ministry of Health and Welfare said, "The submission of PE data can be omitted for rare drugs used for diseases that threaten survival as alternative or therapeutic drugs or anticancer drugs without treatments." The Ministry of Health and Welfare explained, "In addition, the process of revising the regulations of the Korea Appraisal Board, which includes drugs that improve the quality of life of children with anticancer drugs or rare disease treatments, is in progress. In addition, the expansion of rare drugs for infants, children, and adolescents to PE omission is a matter to be carefully considered in consideration of other drugs and equity."
- Policy
- Reyvow, a migraine drug aimed at clinicians
- by Lee, Tak-Sun Aug 11, 2022 06:03am
- Ildong Pharmaceutical, which is seeking to introduce the new migraine drug Reyvow in Korea, has decided not to accept the upper limit offered by the Pharmaceutical Benefit Evaluation Committee of the Health Insurance Review and Assessment Service. The measure was decided in consultation with Lilly, who holds the global copyright of the drug, and they say they will continue to make efforts to register their salaries. On the 7th of last month, the Pharmaceutical Benefit Evaluation Committee of the HIRA Committee reviewed Ildong Pharmaceutical's acute migraine treatment Reyvow 50mg and Reyvow100mg and judged that the benefit was appropriate if it was accepted below the evaluation amount. If Ildong Pharmaceutical accepted less than the evaluation amount, there was a possibility of benefit registration. However, it is said that the amount was not acceptable for pharmaceutical companies. The committee presented a weighted average price of existing treatments, including Tryptan-based drugs, which differed greatly from the price desired by pharmaceutical companies as the prices of existing drugs fell significantly due to the expiration of patents. Reyvow has attracted attention as an expected replacement for Tryptan-based drugs, which are most commonly used to treat migraine headaches. This drug targets serotonin (5-HT) 1F receptors like conventional Tryptane drugs, but it has the advantage of not having cardiovascular side effects by acting selectively. Tryptane-based drugs were limited in their use because they contract blood vessels on the mechanism and cause cardiovascular diseases such as myocardial infarction and stroke. An industry official said, "We needed a migraine treatment that can be used comfortably in clinics because existing drugs have side effects risks," adding, "Reyvow is an oral drug and has a low risk of side effects, so we expected Reyvow to be reimbursed." All procedures were suspended as everyone did not accept the price offered by the HIRA. However, they say they will continue to push for efforts to register benefits in the future. It is known that products will be released in the second half of this year. Reyvow, which was approved by the U.S. FDA in 2019, is a drug that Ildong Pharmaceutical has secured copyrights in eight Asian countries, including domestic sales licenses, in partnership with its developer CoLucid in 2013. When Lilly acquired CoLucid in 2017, the drug's global copyright was also transferred to Lilly. All of them accelerated the introduction of Bridging Study in Korea. Meanwhile, Lilly Emgality and Teva's Ajovy, which are new drugs for the same migraine, are also seeking to register their salaries. In particular, Emgality has completed negotiations with the NHIS and is expected to be listed soon. However, the both are injection typed medication and expensive, so it is not expected to be prescribed much in clinics.
- Policy
- "May bring 60M courses of Omicron-adapted COVID-19 vaccines"
- by Lee, Jeong-Hwan Aug 11, 2022 06:03am
- The disease control authorities announced that they may introduce the adapted, so-called ‘Omicron-specific COVID-19 vaccines’ as newly introduced amounts if the vaccines receive marketing authorization. In other words, if Moderna’s ‘Moderna Spikevax-2 inj.’ and Pfizer’s ‘Comirnaty-2 inj.’ that are being reviewed by the Ministry of Food and Drug Safety are approved, the authorities will bring in these vaccines as newly procured vaccines. On the 9th, the Korea Disease Control and Prevention Agency responded so to a written QA on pending issues related to COVID-19 that was submitted by members of the National Assembly’s Health and Welfare Committee. Adapted vaccines refer to multivalent mRNA vaccines that express antigens for both the original COVID-19 virus (Wuhan strain) and its variant virus (Omicron strain, BA.1) that was developed as a booster vaccine after receiving primary vaccination to be administered in the endemic stage. Moderna Spikevax-2 inj. and Comirnaty-2 inj. are both under review by the MFDS for approval. The KDCA explained that the purchase agreement made with Moderna and Pfizer in 2022 contains the supply of adapted vaccines. If approved, the 60 million courses of vaccines set to be introduced to Korea may all be adapted vaccines. To the question of when the adapted vaccines will be administered, the KDCA said the specific date will be announced at the end of the month after a comprehensive review of the vaccine’s efficacy, development progress, and introduction situation. However, the specific strategies such as vaccination subjects, period, and method would need to be determined based on the variant in circulation at the time of vaccination and the effect of the adapted vaccine against that variant. The KDCA added that it will continue to monitor the development of adapted vaccines in Korea and abroad and cooperate with the MFDS for the prompt introduction of adapted vaccines. Meanwhile, the U.S. companies Pfizer and Moderna are currently developing adapted vaccines that include recently circulating variants. Pfizer is yet to apply for the approval of its vaccine in Korea, and Moderna has completed submitting its application for approval on the 29th of last month.
- Policy
- Will approval of brain function enhancers be revoked?
- by Lee, Hye-Kyung Aug 10, 2022 05:53am
- Whether acetyl-L-carnitine, which failed to verify its efficacy in its ‘secondary degenerative diseases caused by cerebrovascular disease’ indication during clinical reevaluations, will be able to maintain its marketing authorization status will be determined within September this year. The Ministry of Food and Drug Safety issued a Dear Healthcare Professional Letter on the 5th recommending the use of alternative drugs and discontinuing the prescription and dispensing of drugs that contain acetyl-L-carnitine. At the MFDS correspondents’ briefing held on the 9th, Kyung Seung Shin, Director of the Pharmaceutical Safety Evaluation Division at MFDS, said, “We have disclosed the reevaluation results in compliance with our administrative procedure, and will be receiving objection submissions for 10 days starting on the 20th day of the public announcement. When the final result will be announced may vary depending on whether or not an objection is filed and other circumstances." Director Shin said, “The process will likely be completed within September. If the Ministry does not accept the objections submitted by the companies, acetyl-L-carnitine drugs will be recalled, suspended from sales, and revoked of their marketing authorization.” Currently, 39 acetyl-L-carnitine products from 35 companies are sold in the Korean market, with representative products being Dong-A ST’s ‘Dong-A Nicetile Tab.’ and Hanmi Pharmaceutical’s ‘Carnitil Tab.’ Pursuant to Article 33 of the Pharmaceutical Affairs Act, the MFDS ordered related companies to reevaluate and verify the efficacy of acetyl-L-carnitine through domestic clinical trials based on the latest scientific standards. The companies have already failed to demonstrate acetyl-L-carnitine’s efficacy in ‘primary degenerative disease’ in a previous clinical trial that was conducted in June 2019, which resulted in the deletion and removal of the indication. Shin said that the most frequent request made by the companies during clinical reevaluations is to extend the reevaluation period. “The companies wish for more time. We have partially accepted such requests per claims that patient recruitment is difficult due to COVID-19.” However, the ministry takes thorough reports on the interim progress of the clinical reevaluations for the safety of the consumers. Shin said, “Although the companies say that conducting domestic trials within the period is difficult, as their drugs are still being sold in the market during the period, it is our responsibility to confirm the progress for the consumers. We receive interim result reports so as to prevent delays or discontinuation." Regarding the increased burden borne by the industry due to MFDS’s clinical reevaluations and HIRA’s reimbursement evaluations, Shin said, “The companies may feel burdened by the reevaluations being separately conducted by different institutions on one product, but this is just standard procedure that needs to be carried out by each institution." “HIRA conducts reevaluations on the adequacy of a drug’s reimbursement, while the MFDS reevaluates whether the clinical effectiveness of a drug meets the latest scientific standards. Although the institutions closely share their progress, as the reevaluations are conducted for different purposes, their evaluation systems have to be operated separately." Shin also explained the risk management plan system that was included in the new government's regulatory reform innovation plan, which will be operated in integration with the post-marketing safety management system after the abolition of the re-examination system. Shin said, “There had been industry burden of additionally needing to conduct risk mitigation measures if a drug is designated as a drug subject to risk management while conducting mandatory PMS for 4-6 years from a drug’s marketing date. The two laws in effect have many similar and overlapping parts, so a plan was made to integrate the laws into a single risk management system. We will promptly work to amend the Pharmaceutical Affairs Act for this.” Shin also mentioned the pharmaceutical equivalence reevaluations that are set to begin next year and end in 2025. The MFDS recently disclosed the total list of items subject to pharmaceutical equivalence reevaluations for the next 3 years, which includes 130 ingredients such as levodropropizine in 2023, 420 ingredients including amlodipine in 2024, and 286 ingredients including piroxicam in 2025. Shin said, “We plan to receive supplementary documents for items subject to reevaluations this year until the end of the year. Some companies requested deadline extensions due to difficulty generating data, so we may receive data until early next year."
- Policy
- AZ seeks reimb of its triple inhaled therapy for COPD
- by Lee, Tak-Sun Aug 10, 2022 05:52am
- The first single-inhaler triple therapy for COPD in Korea, New triple drug inhaled therapies for COPD (Chronic Obstructive Pulmonary Disease) are working to receive insurance reimbursement in Korea. After GSK cut the first tape to reimbursement with its ‘Trelegy Elipta’ in June last year, AstraZeneca has also applied for the reimbursement of its ‘Breztri Aerosphere,’ which was approved in November last year. According to industry sources on the 8th, AstraZeneca applied for reimbursement of its Breztri Aerosphere to the Health Insurance Review and Assessment Service. Breztri Aerosphere is a single-inhaler, fixed-dose triple-combination of budesonide glycopyrrolate (undifferentiated), budesonide (undifferentiated), and formoterol fumarate (undifferentiated). The inhaler is indicated for the maintenance treatment of moderate-to-severe COPD in patients who are not adequately treated by a combination of a long-acting beta2-adrenergic agonist (LABA) and inhaled corticosteroid (ICS) or a combination of a long-acting muscarinic antagonist (LAMA) and LABA. In other words, this triple therapy emerged as a new alternative for patients with COPD that did not respond well to existing ICS-LABA or LABA-LAMA combination therapies. Triple inhaled therapies have first entered the Korean market with the reimbursement approval of GSK’s Trelegy Elipta in June last year. The insured ceiling price of the drug was set at ₩45,602 for 30-day supply. At the time of listing, its price received attention for being similar to that of existing dual combination therapies. Trelegy Elipta’s price is expected to act as a benchmark for the other drugs that will follow. Triple inhaled COPD therapies that are currently approved in Korea are: Trelegy Elipta, which was approved in 2018,; Breztri Aerosphere, which was approved in November last year; and Kolon Pharma’s Trimbow, which was approved in September 2019. Although Kolon is yet to apply for the reimbursement of Trimbow, AstraZeneca is quickly pushing to reimburse Breztri Aerosphere. AstraZeneca’s hastening the release of its triple therapy combination to prevent GSK's monopoly in the COPD inhaler market. However, the key is price. AstraZeneca cannot help but consider price competitiveness as Trelegy Elipta was listed with reimbursement at a relatively nice price'. Currently, it is estimated that around 50,000 COPD patients in Korea will be in need of treatment with triple inhaled therapies. With so many patients present, the competition between GSK and AZ is expected to heat up in the market.
- Policy
- When replacing Spinraza→Zolgensma, it should be injected
- by Lee, Hye-Kyung Aug 10, 2022 05:52am
- If SMA patients who were administering Biogen's Spinraza want to be replaced with Zolgensma, an ultra-high-priced one-shot treatment from Novartis Korea, they should have a minimum administration interval of 4 weeks. Regardless of whether or not benefit of Zolgensma is applied, replacement administration to other treatments after Zolgensma administration does not recognize the application of benefits. On the 4th, the HIRA guided "Questions and Answers on Zolgensma benefit standards." Zolgensma will be reimbursed from the 1st of this month as the insurance drug price of 1,981,726,933 won per kit was set through the Health Insurance Policy Review Committee on July 20. It is recommended to have an administration interval of at least 4 weeks (ideally 4 months) as there may be additional risks, such as an increase in the number of Zolgensma replacement administration times in Spinraza. Age conditions are judged on a calendar basis. Under 9 months of age means the day before the birth date + 9 months of age, up to 12 months of age / up to 12 months of age (temporary replacement) until the day before the birth date + 13 months. Permanent respiratory use refers to the case of using a ventilator for more than 16 hours a day and more than 14 consecutive days. Submission of consent to implement a long-term follow-up investigation for Zolgensma administration is mandatory for the application of Zolgensma insurance and must be submitted when applying for prior approval. Medical institutions are encouraged to fully explain Zolgensma effects and side effects to patients (caregivers) and to obtain consent from patients and carers before administering this drug.
- Policy
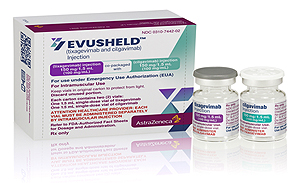
- Administration of Evusheld begins to prevent COVID-19
- by Kim, Jung-Ju Aug 09, 2022 05:57am
- From today (8th), the quarantine authorities will administer Evusheld, an antibody injection for prevention, for severely immunocompromised patients. The COVID-19 Vaccination Response Promotion Team (Director Baek Kyung-ran) announced today (the 8th) that it will start taking Evuseld to prevent COVID-19 among immunocompromised people. Evuseld is a preventive antibody injection that directly injects antibodies into the body to cause preventive effects to those who are unable to form immunity due to immunosuppressive treatment and severe immunodeficiency symptoms. Evuseld was introduced in Korea under the approval of the MFDS. As a result of clinical trials, preventive effects were confirmed, such as 93% reduction in infection and 50% reduction in severe and death. Recently, the Korea Centers for Disease Control and Prevention explained that the effectiveness of BA.4 and BA.5, which are subvariables of omicron, has also been confirmed to be maintained. The most common adverse event among reported adverse reactions in safety was injection site response (2%), mostly mild (73%) or severe (24%), and no particular safety problem was identified. The subjects of the medication are blood cancer patients receiving immunosuppressive treatment, organ transplant patients, and congenital (primary) immunodeficiency patients. This standard was prepared through consultation with related professional societies and experts such as the KSID, The Korean Society of Hematology, The Kosaids, and Korean College of Rheumatology. Currently, there are 35 high-level general hospitals, 99 general hospitals, and 76 hospitals, with 210 designated nationwide, and medical staff must receive medication training such as drug characteristics, medication targets, injection methods, and contraindications. If there is a patient who believes that medication is necessary according to the medication guidelines, reservations and applications can be made through the COVID-19 vaccination management system. When an application from a medical institution is received, the Korea Centers for Disease Control and Prevention will check with the local health center and apply for the allocation of drugs to the relevant medical institution before the scheduled date. The promotion team said, "In order to prevent COVID-19, vaccination is recommended first, but if it is difficult to expect antibody formation even after vaccination, additional protective effects can be expected through Evusheld, a preventive antibody injection."
- Policy
- Oxiracetam’s next...ordeal of brain function enhancers
- by Lee, Tak-Sun Aug 09, 2022 05:56am
- Korean Drug Co.’s It’s a time of ordeal for brain function enhancers. In addition to choline alfoscerate, whose scope of use has been reduced through reimbursement reevaluations, acetyl-L-carnitine failed to demonstrate its efficacy during clinical reevaluations. As a result, acetyl-L-carnitine is expected to completely disappear from the prescription market. Oxiracetam, which accounts for the third-largest share of the brain function enhancer market, is set to receive clinical reevaluations and reimbursement reevaluations soon. If oxiracetam also takes a blow, industry officials predict that the brain function enhancer market itself will greatly retract. On the 5th, the Ministry of Food and Drug Safety announced that it will suspend prescriptions and dispensing of acetyl-L-carnitine as the drug failed to demonstrate its efficacy in ‘secondary degenerative diseases caused by cerebrovascular disease’ during clinical reevaluations. In other words, acetyl-L-carnitine was unable to prove its efficacy in the indication. Acetyl-L-carnitine had previously been unable to demonstrate its efficacy for 'primary degenerative diseases' during clinical reevaluations. Being unable to demonstrate efficacy for both of its indications, the ingredient is set to be removed from the reimbursement list. Acetyl-L-carnitine had been subject to reimbursement reevaluations next year, however, if it is removed from the reimbursement list, the ingredient will naturally be excluded from reevaluations as well. The average annual claims amount of acetyl-L-carnitine over the last 3 years was ₩58.1 billion, the second-best grossing ingredient in the brain function enhancer market next to choline alfoscerate, which boasts a market size of ₩500 billion. The problem is that the next-in-place brain function enhancer is also up for reevaluations soon. The drug up for the next reevaluation is oxiracetam, which is represented in Korea by Korean Drug Co.’s Neuromed tablet. Clinical reevaluation reports for oxiracetam are also due for submission by the end of this year. Like other ingredients, oxiracetam’s indication for improving Alzheimer-type dementia had also been deleted due to the inability to demonstrate its efficacy, and a clinical reevaluation is being conducted to verify its efficacy for the remaining indication, for the 'improvement of vascular cognitive disorder symptoms.’ If the year-end reports submitted are unable to verify the drug’s efficacy in the remaining indication, the prescription and dispensing of oxiracetam will likely be discontinued, just like in acetyl-L-carnitine's case. However, another great obstacle remains even after verifying the clinical efficacy as oxiracetam is also subject to reimbursement reevaluations next year. Oxiracetam’s 3-year average claims amount is ₩23.3 billion, less than that of choline alfoscerate or acetyl-L-carnitine. But as Neuromed raised ₩11.5 billion in prescriptions last year, the amount earned in terms of each item is by no means small. If oxiracetam also suffers a blow from that reevaluation, all the main brain function enhancers will be reduced reimbursement or removed from the reimbursement list, thereby contracting the total market. Brain function enhancers have been widely used to prevent dementia in elderly patients and have well served as a cash cow for domestic pharmaceutical companies. Reimbursement for choline alfoscerate was maintained through the reimbursement reevaluations that were conducted to verify the drug’s efficacy in dementia in 2020. However, the patient coinsurance rate for the main indication in improving cognitive disorders rose from 30% to 80%, and was applied selective reimbursement. Despite this, the previous reimbursement rate is being applied because some of the affected companies appealed the decision, and filed for a stay of execution of the reimbursement reduction disposition. However, Chong Kun Dang and the other companies lost the suit that they filed to cancel the reimbursement reduction, and the stay of execution will be lifted soon. Also, the fact that the companies must demonstrate their drug’s efficacy through clinical reassessments by 2025 will add a significant burden on the companies.
- Policy
- It's hard to prescribe oral medicine for COVID-19
- by Kim, Jung-Ju Aug 09, 2022 05:56am
- Amid the remarkable re-proliferation of COVID-19, the newly proposed National Infectious Disease Crisis Response Advisory Committee advised medical staff to create detailed guidelines for prescriptions in consideration of difficulties in the dispensing process. The National Advisory Committee on Infectious Disease Crisis Response (Chairman Chung Ki-seok) held the 3rd National Advisory Meeting on Infectious Disease Crisis Response on the 4th to discuss such issues. The third meeting was attended by 21 members of the National Advisory Committee on Infectious Disease Crisis Response, including Chairman Chung Ki-seok, the Central Disease Control Headquarters, and the COVID-19 Vaccination Response Promotion Team. The advisory committee first said that in-depth analysis of related cases should be added to establish a data-oriented quarantine policy as re-infected cases and deaths of children and adolescents are being confirmed in relation to the current epidemic. In the prediction of trends, it was asked to create a system that allows a number of research teams to conduct systematic mid- to long-term modeling research that reflects various policy effects beyond supporting the results of mathematical modeling-based predictions. In the case of PO treatments, the operation status of the currently operating one-stop medical institution was checked so that patients visiting the hospital could be prescribed and treated in a timely manner to discuss the need for a system in which all citizens are guaranteed proper COVID-19 treatment services without inconvenience. In particular, the advisory committee said that guidelines should be drawn up so that medical institutions can pay close attention to the on-site procedures and processes of pharmacies that prepare eating treatments. In fact, unlike ordinary dispensing patients in pharmacies, the preparation and medication guidance for COVID-19 oral preparation patients are complicated, and the billing method is differentiated from other health insurance, so it takes a lot of time and requires concentration. In response, the advisory committee reviewed, "The government should also try to expand medical institutions and pharmacies, but it is necessary to produce and distribute detailed prescription guidelines to medical staff so that they can help in the medical field." He suggested that the system needs to be checked and prepared so that children wishing to be vaccinated can be vaccinated quickly as the number of COVID-19 children and adolescents has increased since the Omicron epidemic.