- LOGIN
- MemberShip
- 2025-12-23 12:55:55
- Policy
- Drug prices are the key to easing the PAH benefit standard
- by Choi-sun Oct 13, 2021 05:46am
- There is a possibility that PAH tx benefit standard, which has been criticized by strict standards, will be eased. As the HIRA mentioned that it has prepared an amendment that fully reflects the opinions of the society on the application indicators of combination therapy and the initial three-drug combination pointed out as a problem, the public has passed over to the MOHW. On the 15th, the Korean guideline for diagnosis and treatment of treatment and the Korean Pulmonary Hypertension Association held a National Assembly debate with Heo Jong-sik, a member of the National Assembly's Health and Welfare Committee, to establish measures to improve the survival rate of patients with PAH. In Korea, the three-year average survival rate of patients with PAH is only 54.3%, ranking the lowest among OECD countries, and very low compared to 82.9% in Japan and 73% in the United States. The main reason for this is that unlike global treatment guidelines, which are recommended to use combination therapy from the beginning, combination therapy is only possible in high-risk groups in Korea. As a result of The Korean Society of Cardiology and the Korea Lung Arterial Hypertension Patients Association filing complaints about the revision of standards, the HIRA held an expert advisory committee with related societies in July to discuss the validity of the revision. Pulmonary hypertension, a rare disease, improves the prognosis if diagnosed quickly and treated appropriately. As the mortality rate varies significantly depending on the active use of drugs in the early stages, the association requested to ease the indicator criteria for combination therapy and increase the choice of combination therapy drugs from the beginning. Park Jae-hyung, a cardiology professor at Chungnam National University Hospital, said, "The problem with the treatment of pulmonary hypertension in Korea is that the treatment is delayed because the conditions for starting the combination therapy are high," adding, "Please lower the standard and lift the restrictions on the choice of combination therapy." In this regard, the HIRA also said it reflected the opinions of academia and patient associations as much as possible. Ha Sung-hee, head of the drug standard department at the HIRA Drug Management Office, said, "There are not many diseases in which domestic treatment guidelines are made, but it is quite encouraging that domestic treatment guidelines have been established for rare diseases such as PAH." She said, "Based on this, we had a meeting with experts from the society in July to prepare an amendment to the benefit standards, and we organized the contents and reported them to the MOHW." In the debate, questions flooded about the exact timing of improvement in the standard because the survival rate within three years was only half. "We need to take a close look at various financial capabilities," Ha said adding, "We know that many people are waiting because there are follow-up procedures such as administrative notices to announce, but please understand that it takes time." The MOHW also suggested the possibility of expanding the salary standard through drug price negotiations with pharmaceutical companies. Yang Yoon-seok, head of the insurance drug division at the MOHW, said, "As the association has created medical guidelines, it seems desirable to meet health insurance benefits as much as possible." He said, "We are looking at the revision that came up after a meeting of the expert advisory committee in July." He said, "We will review from the patient's point of view how those suffering from the disease can be properly treated." He explained, "We are reviewing whether to expand benefits based on cost effectiveness, and we think PAH needs measures not only in drugs but also in the areas of diagnosis and follow-up management."
- Policy
- Hanmi's HM15912 is approved for phase 2 in Korea
- by Lee, Tak-Sun Oct 12, 2021 05:50am
- Hanmi Pharmaceutical is developing a rare disease treatment aimed at the global market and is conducting phase 2 clinical trials in Korea. With HM15912 as a candidate for the treatment of Short bowel syndrome (SBS), clinical trials will be conducted at Samsung Medical Center. The MFDS approved Hanmi's phase 2 clinical plan for HM15912 on the 8th. This is a phase 2 clinical trial (DOLPHINS-2) to evaluate the safety, pharmacokinetics and pharmacokinetics of HM15912 in adult subjects with SBS-IF. A total of seven patients will participate in this test, of which two are domestic. Short bowl syndrome (SBS) is a disease in which the small intestine is naturally short in length or more than 60% of the small intestine is lost by surgical resection. This is known to cause rapid malnutrition due to food absorption. It is a rare disease in which less than five people occur per 100,000 people. Patients receive nutrients through blood vessels, which makes normal daily life difficult and can cause side effects such as liver failure and thrombosis in the long run, so treatment is urgent. Hanmi Pharmaceutical is developing this drug in the global market. In particular, Hanmi Pharmaceutical's own technology, the Labscovery platform, is being developed as a formulation drug administered once a month. Earlier this year, phase 2 clinical trials were approved by the U.S. FDA and began clinical trials in June. The U.S. FDA and the MFDS have designated this drug as a rare drug in the development stage to support rapid commercialization. If designated as a rare drug by the FDA, benefits such as tax reduction, exemption from the cost of applying for permission, and exclusive rights for seven years upon approval for the first time among products of the same affiliate will be granted. Few patients are suffering from rare disease treatments, but global pharmaceutical companies are also focusing on development due to high costs. Rare drugs with high effectiveness receive high value. If Hanmi Pharmaceutical proves its validity in phase 2 clinical trials, it is also expected that overseas big perm will buy it. Attention is focusing on whether South Korea and the U.S. will be able to reach the global stage through their own technology in the rare disease treatment market.
- Policy
- Gov “It was the experts’ decision to not list Kymriah”
- by Kim, Jung-Ju Oct 12, 2021 05:49am
- In response to NA’s criticism regarding the delayed reimbursement listing of Kymriah, the government has expressed its difficulties as the decision was a result of an expert assessment. Also, the government denied the claim that that insurance coverage is only being extended for mild diseases. This was the response Health and Welfare Minister Deok Cheol Kwon gave to the claims that were made by NA member Jong Sung Lee at the NA Health and Welfare Committee’s audit of the Ministry of Health and Welfare that was held on the 7th. Lee had previously pressed the government for the prompt listing of Kymriah, using Soliris in 2012 and Spinraza in 2019 as examples of how the government is passive in listing new drugs. Lee said, “A clinical trial had shown that Kymriah could increase the survival rate by 60%. It feels that the government is immersing itself in populist policies - those that benefit a large number of mild patients - and neglecting the small number of severely ill patients.” He continued, “How hard could it be for the government, which had once announced that it could spend 1 trillion won as phone bill support for the public, list one single drug for our young patients?” Minister Kwon immediately refuted Lee’s claim. Kwon said, “The claim that only the coverage for mild disease patients has increased during President Moon's administration is not true. The coverage rate of severe disease patients is currently over 70%. I believe there is a misunderstanding here. The government also believes that we should promptly provide insurance benefit for severely ill patients.” He explained that the decision on Kymriah was not made by the government and was a result of an expert assessment. “Kymriah is an ultra-high-priced drug, and is being reviewed by the experts.”
- Policy
- The MOHW expressed reluctance to Keytruda's primary benefit
- by Lee, Jeong-Hwan Oct 12, 2021 05:49am
- Minister Kwon Deok-cheol (photo = provided by the National AssemblyMinister of Health and Welfare Kwon Deok-cheol expressed reluctance to applying MSD immuno-cancer drug Keytruda as a primary treatment for lung cancer, citing enormous health insurance financial needs. On the 6th, Minister Kwon responded to a question from Kang Sun-woo (the Democratic Party of Korea) at the National Assembly Welfare Committee's parliamentary audit. He pointed out that when lung cancer was first treated with Keytruda, it was more effective in treating diseases than when used as a secondary treatment. He introduced that the U.S. NCC guidelines also recommend Keytruda as a priority in single and combined therapy. He criticized that unlike overseas countries such as the United States, Korea is applying benefits so that Keytruda can only be used as a secondary treatment in the event of a traditional chemotherapy failure, increasing the burden on patients. He said, "It costs 7 million won a month for lung cancer patients to spend Keytruda at their own expense from the first treatment. He said,"if it is administered every month, about 100 million won per year is needed. 52 countries around the world have already applied the primary benefit, and 31 OECD countries have also recognized the primary benefit." He then asked Minister Kwon Deok-cheol if he had any plans, such as a system that is registered first and then evaluated for Keytruda benefits or a separate cancer fund. Minister Kwon replied that the pre-registration & post-evaluation system is also under comprehensive review in consideration of the financial burden of health insurance and difficulties in managing drug prices. He explained, "If Keytruda, the current secondary treatment, is allowed as the primary treatment, it will affect huge health insurance finances, so there should be a (cost-effectiveness) evaluation." He said, "In the system, it becomes reasonably difficult to manage drug prices because the health insurance finance has to pay the drug price at the time of pre-registration." "We need to comprehensively review that," he added.
- Policy
- Pfizer vaccines from Romania not transported in official box
- by Lee, Jeong-Hwan Oct 08, 2021 05:56am
- Criticism has been raised that the 1,053,000 doses of Pfizer’s COVID-19 vaccine that the government received from Romania last month were not stored in transport boxes that were officially authorized by its manufacturer, Pfizer. Pfizer’s vaccines require ultra-low-temperature storage for quality maintenance and should be stored in Pfizer’s custom shipping box that ensures cold chain storage. However, the criticism is that the government used private boxes, which could have compromised the quality of the vaccines. On the 7th, National Assembly member Jong Hean Baek of the People Power Party raised the issue based on the data submitted by the Korea Disease Control and Prevention Agency. According to the data, while transporting Pfizer's vaccines from Romania to Korea via Incheon airport in September, the Korean government used vaccine shipment boxes provided by a separate private transport company. The background is that the Romania government had returned Pfizer’s custom shipment back to Pfizer after directly purchasing the vaccines. Baek said that using private storage boxes for sensitive vaccines that should be transported in specially designed ultra-low-temperature storage containers is the issue. Although they look like regular delivery boxes, Pfizer’s custom boxes use new plastic material or polymeric compound-coated corrugated cardboard and are strong against moisture, and have excellent thermal insulation. Baek added that Pfizer meticulously packs its vaccines in custom boxes in its Belgium facility before shipping them to Europe. This is to maintain the ultra-low temperature required to ensure the quality of the vaccine, and the box is packed in two layers and filled with refrigerants in between. Baek added that in Korea, Pfizer Korea receives and the vaccines from the custom shipment boxes, takes out the vaccines, and returns the custom boxes to Belgium for reuse. In other words, Baek’s view is that the vaccines should have been shipped after obtaining Pfizer's custom shipment boxes to maintain the vaccines’ quality and warranty. As of the 5th, 4,031 cases of adverse events were reported after being vaccinated with the Pfizer vaccine provided by Romania. The government is investing in the causality of the events and said that the vaccines’ temperature has been well maintained in the course of bringing the vaccines to Korea. Baek said, “While conducting the casualty investigation for the Pfizer vaccines from Romania, the government should analyze the cause by looking specifically at whether the initial cold chain was compromised in any way. Also, the government should transparently disclose whether the manufacturer will warrant after-sale service for Romania’s vaccine quality issue.”
- Policy
- Expanding the submission of opinions reflecting permission
- by Lee, Tak-Sun Oct 07, 2021 05:54am
- Opportunities for submitting opinions from industries will be expanded when reflecting permits based on the results of the reexamination. A pre-announcement procedure is added to the previous opinion inquiry. The MFDS announced that since the 27th of last month, the procedure for reflecting permits based on the results of the reexamination has been improved and applied. There was a 14-day period of submitting opinions through "Inquiry on Opinions on Permit Change" before reflecting the results of the retrial in the permission. An opportunity to submit opinions will be given one more time. An official from the MFDS said, "It is expected that opportunities for the industry to submit opinions will expand through a pre-announcement period." A total of 28 days of opinion submission period will be given. At the end of the pre-announcement period, a change order will be issued as before. After the change order, the changes in permission matters within three months must be reflected in the product insert. In this improvement plan, in order to distinguish between PMS survey results and general post-marketing adverse effect analysis evaluation, it will be divided into domestic post-marketing adverse effect analysis results and domestic post-marketing adverse effect analysis evaluation results. The results of the analysis and evaluation of the adverse effect after domestic marketing are the same as the existing re-examination method. An official from the MFDS explained, "We distinguish between the results of the adverse effect analysis collected in the post-market survey and the results of the adverse effect analysis reported after the market and reflect them in the permit, but we revised the phrase to clarify the analysis results." The improvement of the method of writing the improvement plan will be applied from the change of permission after the 27th of last month, and the pre-announcement period will be given from the items for re-examination received after the same day.
- Policy
- ’With Covid’ scheme unclear with over 5,000 cases expected
- by Lee, Jeong-Hwan Oct 07, 2021 05:53am
- Concerns have been raised that Korea will be unable to adopt the ‘With Corona’ scheme due to the public’s distrust in vaccinations, the government’s non-acceptance of casualties of adverse reactions from vaccines, and the surge in daily COVID-19 cases, etc. Based on the mathematical model that took into account current incidence, the transmission of COVID-19, and the vaccination rate, the authorities expect that the number of new COVID-19 cases may increase on average up to 5,000 cases per day if the fourth wave of the COVID-19 pandemic persists. The National Assembly member Jong Hean Baek of the People Power Party announced the above results on the 6th based on the data submitted by the Korea Disease Control and Prevention Agency. According to KDCA data, if the current incidence, transmission, and vaccination rate are applied to a mathematical model, around 5,000 new COVID-19 cases are expected to arise per day. As for the reason, Baek pointed to the public distrust in vaccines despite inoculation being required for the living ‘With Corona’ scheme, the government’s non-acceptance of casualties of adverse reactions from vaccinations, and the lack of responsibility of the nation in taking care of those who suffer from misinoculations or side effects pre- and post-vaccination. The actual recognition rate of vaccine’s side effects was 53.4% in general patients and a mere 0.3% in deaths. More specifically, KDCA’s immunization surveillance investigation team’s assessment report showed that causality of 1,764 in 3,305 cases, or 53.4% of the cases were recognized. However, among the 678 cases of deaths after vaccination, the causality of only 2 was recognized. Also, 2,014 cases of vaccine misinoculations arose due to the government's neglect, but none were compensated for the damages. Baek said, “To live ‘With Corona’, we must first address the public’s distrust in vaccinations and prepare post-management measures. The citizens are anxious and disappointed by the government’s irresponsible response to the deaths from vaccinations.” He added, “I do not understand why the government is irresponsible in dealing with this life-threatening issue for administrative convenience. The government needs to show responsibility so that the people can trust the authorities and receive their vaccinations.”
- Policy
- Nexviazyme has been applied for domestic permission
- by Lee, Tak-Sun Oct 07, 2021 05:53am
- Sanofi plans to release a new Pompe's disease treatment in Korea. It is known that Nexviazyme, which was approved by the U.S. FDA in August, recently applied for permission from the MFDS. According to the MFDS on the 1st, Sanofi Aventis Korea submitted a report on the results of clinical trials by Nexviazyme (Avalglucosidase alfa-ngpt) and applied for permission. This drug is a Pompe's disase treatment. Pompe's disease is a genetic disorder in which respiratory failure and myocardial disease appear due to muscle strength loss and muscle atrophy. It is a rare disease that is reported to be about one person per 40,000 people worldwide, and it is known that there are about 1,300 patients in Korea. Pompe's disease is divided into IOPD, a fast-onset infant disease in infancy, and LOPD, a late-onset disease that develops at all ages and gradually weakens muscles. As muscles are damaged, heart failure, respiratory failure, exercise disorders, and sleep disorders also occur. The disease administers drugs targeting M6P receptors that allow GAA enzymes to migrate to intracellular lysosomes to treat GAA gene abnormalities. Existing treatments include Sanofi's Myozyme. Nexviazyme, which applied for permission this time, reportedly increased the M6P content by about 15 times compared to Myozyme. Nexviazyme was approved by the U.S. FDA in August. The MFDS designated the drug as a rare drug for patients to use before it was officially approved in June. If designated as a rare drug, the drug can be purchased through the KODC.
- Policy
- Introduction of pre-registration is difficult
- by Kim, Jung-Ju Oct 07, 2021 05:53am
- The government said it is difficult due to concerns over weakening NHIS' drug price negotiation power, while various fields are proposing the introduction of a system that is first registered and evaluated later for access to treatments for severe rare and intractable diseases. Regarding referring to Korean drug prices such as China, the government also said it is actively responding by expanding RSA to prepare for "China risks" such as "Korea Passing." The MOHW recently submitted the "2020 National Assembly Processing Results Report on the Requirements for Correcting and Processing the National Assembly" with such contents. First of all, the National Assembly previously demanded the MOHW to consider introducing a "pre-registration and post-evaluation system" to access treatments for severe rare and intractable diseases. The MOHW said that it is difficult to operate a realistic system for post-registration evaluation. The MOHW replied, "It is expected that it will be difficult to operate a realistic system and manage reasonable drug spending, so careful review is needed." This is because it is difficult to adjust drug prices if pharmaceutical companies do not accept the evaluation results after being registered, and the NHIS' drug price negotiation power may weaken, causing problems. They would expand RSA on countermeasures against so-called "Korea Passing" concerns due to China's drug price system. Overseas countries such as China are deciding their own drug prices by referring to Korean drug prices. Due to the current strict drug price system in Korea, it can lead to " In response, the MOHW replied, "We have introduced RSA since 2014 to respond to delays in insurance registration due to reference to foreign drug prices," adding, "We have been expanding the applicable drugs since October 2020." Regarding the demand to raise funds through the National Health Promotion Fund and the primary benefit of the immuno-cancer drug Keytruda, he replied in July that the HIRA is in the process of expanding benefits of Keytruda and Tecentriq. However, the MOHW said that the National Health Promotion Fund needs to be carefully reviewed by comprehensively considering the target, scope, and allocation of required resources within the scope of support.
- Policy
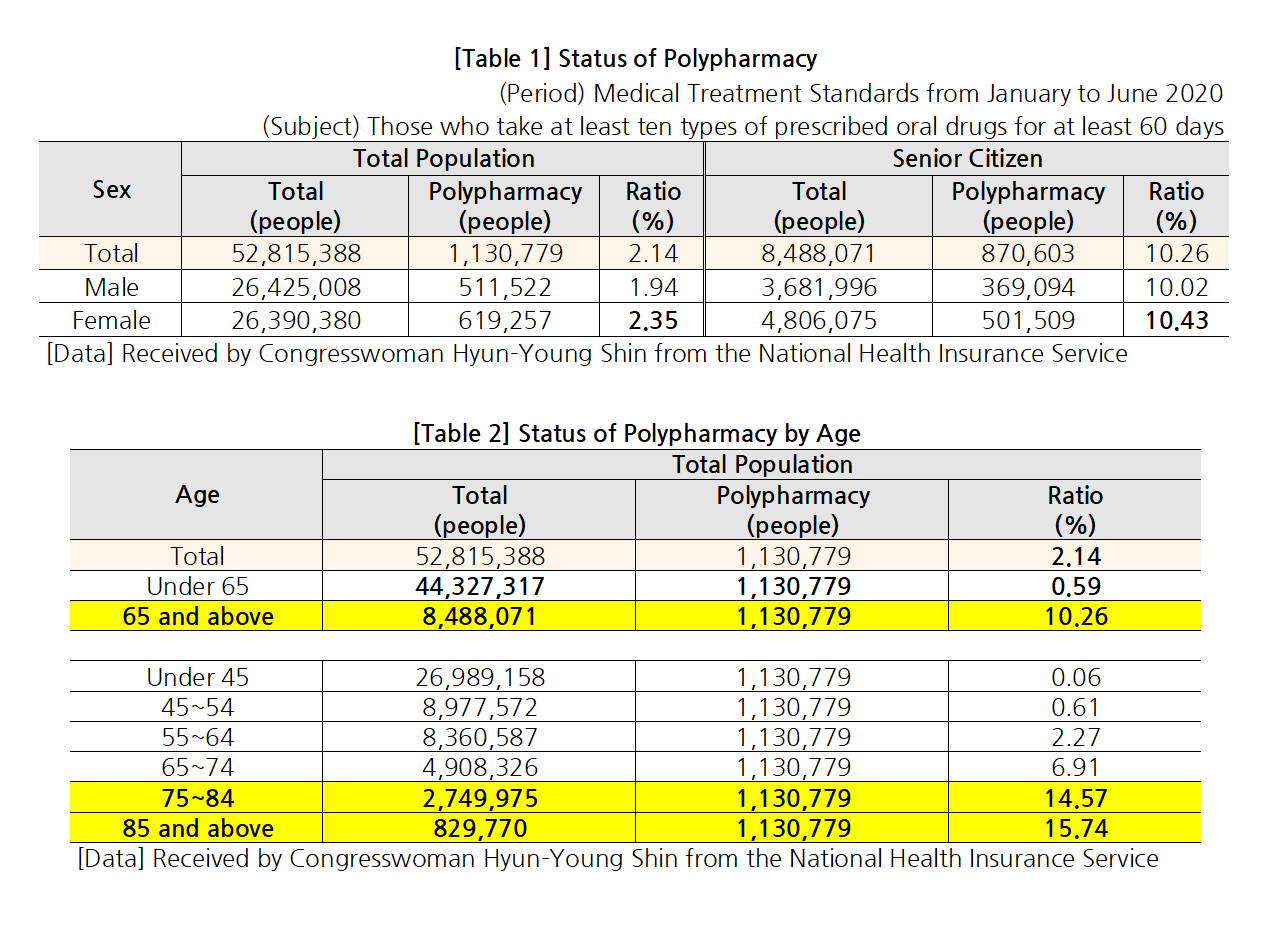
- “Elderly polypharmacy rate Korea 70% vs OECD countries 48%"
- by Lee, Jeong-Hwan Oct 06, 2021 06:06am
- Statistics showed that a high rate - 70.2% - of older adults (aged 75 or over) in Korea chronically take ‘more than 5 drugs for over 3 months.’ The average of 7 OECD countries other than Korea that submitted the same data was only 48%. In other words, concerns over the current polypharmacy status of elderly patients in Korea have been reaffirmed. On the 4th, NA member Hyung-young Shin of the Democratic Party of Korea announced the results above after analyzing OECD data. The medication rate of elderly patients in Korea has been globally high. According to the OECD’s data on the ‘rate of patients aged 75 or over who chronically take 5 or more drugs for over 3 months,’ the average of the 7 countries that submitted the data was 48.3%, compared to the much higher rate of 70.2% in Korea. However, Using an unnecessarily high amount of drugs in older adults can have a negative impact on their health. According to the National Health Insurance Service Ilsan Hospital's report, patients aged 65 or older who take 5 or more drugs have an 18% increased risk of hospitalization and 25% increased risk of death than those who take 4 or fewer drugs. According to the data that Shin received from the NHIS, 2.14% of the total population (1.13 million) in Korea takes 10 or more drugs. Among those, the elderly accounted for the largest proportion with 10.26%, and the polypharmacy rate was higher in women (2.35%) than men. The rate and number of patients using multiple drugs also increased with age. The polypharmacy rate was 10.26% for people over 65 but 15.74% for people over 85. By insurance premium quintile, polypharmacy rate was higher (12.52%) in medical aid beneficiaries than NHI policyholders. The polypharmacy rate in elderly medical aid beneficiaries was 22.57%, which roughly translates to one in 4 to 5 patients. Also, the proportion of the elderly was highest in the 1st and 10th insurance premium quintile (9.88%). Furthermore, the polypharmacy rate was higher in patients with underlying diseases such as diabetes, heart disease, cerebrovascular disease, asthma/COPD, chronic renal failure, pulmonary tuberculosis, etc. By population, the polypharmacy rate was highest in patients with chronic renal failure (18.38%), heart disease, (15.36%), cerebrovascular disease (13.86%). The order was the same in elderly patients but at a higher rate - chronic renal failure (23.80%), heart disease (20.97%), cerebrovascular disease (18.31%). Shin said, “As the rate of patients who take 10 or more drugs increases with age, we need to review whether the patients are taking unnecessary drugs due to over-prescription or redundant prescriptions. In other words, institutional support on the appropriate use of drugs is needed, and a system that assigns primary care physicians for the elderly should be introduced to manage the elderly’s use of multiple drugs, as well as provide customized support on the appropriate use of healthcare.”