- LOGIN
- MemberShip
- 2026-03-10 19:59:46
- Policy
- Bill banning reverse payment agreements passes committee
- by Lee, Jeong-Hwan Aug 28, 2025 06:10am
- The amendment to the National Health Insurance Act, which regulates "reverse payment agreements" where original drug companies and generic drug companies collude by exchanging money to delay or not release generics, thereby avoiding a price reduction for the original drug, passed the National Assembly Health and Welfare Committee review on the morning of the 27th. The illegal collusion among pharmaceutical companies to withhold generic drug launches has forced patients to pay unfairly high prices for medications. This practice must be eradicated to eliminate such disadvantages and prevent unnecessary leakage of health insurance funds. The bill on revising the Pharmaceutical Affairs Act, which expands the post-notification system for generic substitution at pharmacies to the information system established and operated by the Ministry of Health and Welfare and the Health Insurance Review and Assessment Service, and improves the definition of essential medicines and expands support to include drugs with no substitutes or facing supply instability, has also cleared the Health and Welfare Committee. These bills will take effect according to the implementation date specified in the supplementary provisions once they pass the plenary session after review by the Legislation and Judiciary Committee and are promulgated by the government. Original-Generic Pharma Company Collusion Prohibition…Price cuts or reimbursement suspensions applied for offenses The core provision of the National Health Insurance Act amendment bill proposed by Democratic Party of Korea lawmaker Young-seok Seo stipulates that if the Fair Trade Commission uncovers collusion where a generic drug company agrees not to launch a generic in exchange for exclusive domestic distribution rights from an original drug company, the price of the unfairly traded drug will be reduced or its reimbursement suspended. The bill passed by the Health and Welfare Committee amends Article 41-2 (Reduction of Upper Limit Amount for Reimbursement Costs for Drugs, etc.) of the National Health Insurance Act. Specifically, the bill stipulates that for cases violating Article 40(1) or Article 45(1) of the Monopoly Regulation and Fair Trade Act, where the violation was committed “for the purpose of increasing or maintaining the reimbursement price ceiling for drugs,” the insurance price of the drug can be reduced or reimbursement suspended. The amendment allows for a maximum 20% reduction in drug prices when a reverse payment agreement violation is first detected. If another reverse payment agreement is confirmed within 5 years of the price reduction, the price can be cut by up to 40%. If a second violation of the reverse payment agreement is detected within five years after the second price reduction, the application for reimbursement for the drug can be suspended for up to one year. The effective date for the provisions preventing reverse payment agreements is ‘the day six months after the government's promulgation’. Consequently, collusive reverse payment agreements will now face the same level of penalties as illegal pharmaceutical rebates, including drug price cuts and reimbursement suspensions. The Health and Welfare Committee explained, “This bill enables the reduction of the maximum reimbursement amount for drugs related to unfair joint conduct or unfair trade practices, and the suspension of reimbursement coverage. It will prevent pharmaceutical companies and others from profiting through unfair joint conduct or unfair trade practices and establish a fair drug sales order.” Post generic substitution notification system expanded to HIRA A revision to the Pharmaceutical Affairs Act, proposed by Democratic Party of Korea lawmakers Young-seok Seo, Su-jin Lee, and Byung-duk Min, was passed as an alternative bill by the Health and Welfare Committee. It expands the pharmacists’ post-dispensing notification system to the information system operated by the Health Insurance Review and Assessment Service (HIRA). The bill incorporates the existing regulation requiring pharmacists to inform patients when substituting a drug listed on a prescription with an item recognized by the Ministry of Food and Drug Safety as bioequivalent (generic substitution) and to notify the prescribing physician or dentist within one day (or three days if unavoidable circumstances exist). The key provision approved by the Health and Welfare Committee is the establishment of a new Article 27-2 (Establishment and Operation of a Substitution Dispensing Information System) in the Pharmaceutical Affairs Act, enabling the Minister of Health and Welfare to establish and operate an information system to support post-notification of generic substitution.. Notably, the bill allows the Minister of Health and Welfare to delegate this task to the Health Insurance Review and Assessment Service (HIRA), with the necessary details to be stipulated under the Ministry of Health and Welfare ordinance. The effective date for the simplified generic substitution regulations is set for five months after the government's promulgation. This establishes the legal basis for the Ministry of Health and Welfare and HIRA to build and operate a generic substitution information system to support post-notification of generic substitution under the Pharmaceutical Affairs Act. It is expected that this will enable more efficient communication of generic substitutions while clarifying the accuracy of such notifications, thereby enhancing information sharing between physicians and pharmacists. Expansion of the Definition of Essential Medicines & Legal Basis for the National Essential Medicines Stable Supply Council The Committee also passed a revision to the Pharmaceutical Affairs Act that improves and expands the definition of essential medicines to include those without substitutes or facing supply instability, and elevates the legal basis for the National Essential Medicines Stable Supply Council from a presidential decree to a law. The bill provides a detailed definition of essential medicines. These include medicines essential for maintaining the national health system, such as disease control and radiation disaster prevention (Item a), and medicines that are essential for healthcare but lack substitutes or face supply instability despite market mechanisms (Item b). Furthermore, a new Article 83-5 (National Essential Medicines Stable Supply Council) was added to the Pharmaceutical Affairs Act. This mandates the establishment of a National Essential Medicines Stable Supply Council within the Ministry of Food and Drug Safety (MFDS) to develop countermeasures for both national essential medicines and medicines that, while not designated as such, require stable supply due to temporary increases in demand. Notably, the composition of the National Essential Medicines Supply Council, previously chaired solely by the MFDS Vice Minister, was expanded to include ‘one senior official from the Ministry of Health and Welfare designated by the Minister of Health and Welfare,’ bringing the total to two chairs. This appears aimed at strengthening national management capabilities by adding the Ministry of Health and Welfare to the MFDS as a government ministry with authority over national essential medicines. Furthermore, the legal basis for the composition of the council members was clarified. The effective date of the bill was set as one year after its promulgation. While the definition of essential medicines is being expanded, the government's basis for supporting medicines without substitutes and medicines with unstable supply requiring stable supply due to temporary increases in demand is expected to be strengthened.
- Policy
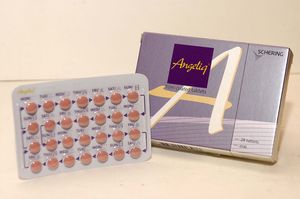
- Reimb listing approved for the first generic of 'Angeliq'
- by Lee, Tak-Sun Aug 27, 2025 06:07am
- Bayer The first generic of Angeliq Tab (drospirenone·estradiol, Bayer), a hormone-based medicine for use in postmenopausal women, will be included in the reimbursement listing. Analysis suggests that the commercialization of domestically produced generic is significant, considering that there had been supply issues related to Angeliq, which is an imported medicine. According to industry sources on August 26, Dalim BioTech's 'Anzeno Tab' will be added to the reimbursement list on September 1 with a ceiling price of KRW 5,565. The product is manufactured directly by Dalim BioTech. Anzeno Tab is a generic drug with the same active ingredients as Bayer's Angeliq Tab. Angeliq is approved for ▲Hormone replacement therapy for estrogen deficiency in women who are at least one year post-menopause, and for the ▲Prevention of osteoporosis in post-menopausal women who are intolerant of or have contraindications to other approved drugs and have an increased risk of fracture. Based on 2024 UBIST data, Angeliq's outpatient prescription sales amounted to KRW 12 billion. Because it is a hormonal drug requiring a separate manufacturing facility had previously prevented generic products from entering the market. Consequently, when supply issues arose with the imported original drug, Angeliq, pharmacies had no identical alternatives, leading to significant difficulties. Alternative prescriptions with similar drugs were the only option, resulting in a continuous demand for generic development. In 2021, the persistent shortage of Angeliq due to production delays at Bayer's Berlin plant caused difficulties for pharmacists. With the launch of this in-house-manufactured generic, the issue of supply instability is expected to be largely resolved. The price of Anzeno Tab was set at KRW 5,565, which is 53.55% of Angeliq's price, without any additional premium, as it met all the required criteria. Angeliq Tab's current ceiling price is KRW 10,393.
- Policy
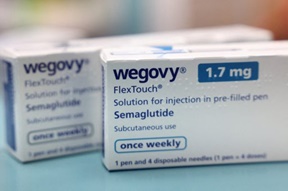
- ‘80,000 Wegovy prescriptions issued per month’
- by Lee, Jeong-Hwan Aug 27, 2025 06:06am
- The popular obesity drug Wegovy, which was launched in Korea in October last year, has already been prescribed about 400,000 times within eight months, raising concerns over potential misuse and abuse. On the 25th, Rep. Kim Sun-min of the Rebuilding Korea Party disclosed the ‘Annual and Monthly Status of Wegovy Prescriptions based on DUR’ data submitted by the Health Insurance Review and Assessment Service (HIRA) and urged safe usage. According to the data, in 2023, the first month of release, the number of prescriptions flagged by the Drug Utilization Review (DUR) system for Wegovy was 11,368. This increased to 16,990 prescriptions the following month, November. The number then reached 21,457 prescriptions in December. This year, the numbers have increased by over 10,000 cases every month to record 22,051 in January, 31,512 in February, and 47,597 in March. Prescriptions then surged to 70,666 in April, then 88,895 in May. In June, the number slightly decreased to 84,848. This means that since its launch in October last year, Wegovy prescriptions averaged 43,931 per month, rising to an average of 57,594 per month this year. Saxenda prescriptions also showed a similar pattern. The number of Saxenda prescriptions in Korea, which was launched in 2018, was 138,353 in 2022, 171,230 in 2023, and 205,109 last year. Experts have pointed out that while these figures alone cannot determine the exact number of recipients—as one patient may have received multiple prescriptions—the steep increase in numbers clearly indicates a rising trend in the number of people receiving injections. Rep. Sun-min Kim stated, “The number of prescriptions via DUR data is exceeding 80,000 per month. Given the recent craze for obesity treatments, we expect significantly more people are actually receiving prescriptions.” Rep. Kim emphasized, “Given the frequent reports of adverse events, obesity treatments must be used safely under the thorough care of specialists.”
- Policy
- MFDS will intensively monitor Wegovy and other obesity drugs
- by Lee, Hye-Kyung Aug 26, 2025 06:05am
- The Ministry of Food and Drug Safety (MFDS) has designated ‘Wegovy’ and other GLP-1-class obesity injectables as targets for intensive monitoring and plans to continuously monitor side effects in collaboration with the Korea Institute of Drug Safety & Risk Management. Th MFDS designates the intensive monitoring status to drugs with high safety concerns in Korea and abroad, or those of high social interest due to concerns about misuse, and intensively collects, analyzes, and reevaluates their adverse events. On the 25th, the MFDS emphasized that injectable GLP-1 class obesity drugs, which are currently attracting significant public attention, should only be used under the supervision of medical professionals for patients diagnosed with obesity, and only in accordance with the approved indication per prescription. Injectable GLP-1 class obesity drugs are prescription drugs indicated for adult obese patients with an initial body mass index (BMI)* of 30 kg/m2 or higher, or adult overweight patients with a BMI of 27 kg/m2 or higher but less than 30 kg/m2 and one or more weight-related comorbidities such as hypertension. According to clinical trial results, even when used within the approved indications, gastrointestinal adverse reactions (nausea, vomiting, diarrhea, constipation, etc.) and injection site reactions (rash, pain, swelling, etc.) are common, and serious adverse effects such as hypersensitivity reactions, hypoglycemia, acute pancreatitis, cholelithiasis, and fluid loss may occur. Also, some medications are contraindicated in patients with underlying conditions such as thyroid medullary cancer, so consultation with a healthcare professional is mandatory. In patients with type 2 diabetes, hypoglycemia and retinopathy may occur, so caution is particularly advised when administering such drugs to patients with relevant medical history. Obesity treatments are prescription-only drugs that must be used under a doctor's prescription with the guidance of a pharmacist. The MFDS urged the public not to purchase or distribute these medications through online platforms, overseas direct purchases, or personal sales. Additionally, the Ministry of Food and Drug Safety recently urged companies holding the marketing authorization rights for the relevant products to prevent misuse and prohibit false advertising, and informed medical professionals about the proper use of obesity treatment medications within their approved scope. The MFDS plans to publish an informational brochure (leaflet) in collaboration with the Korea Institute of Drug Safety & Risk Management, outlining the conditions for which obesity treatment drugs are used, proper administration methods, storage and disposal instructions, precautions during administration, and procedures for reporting adverse reactions (side effects). The MFDS stated, "We will continue to strengthen the provision of information to improve users' understanding of obesity treatment drugs and ensure their safe use."
- Policy
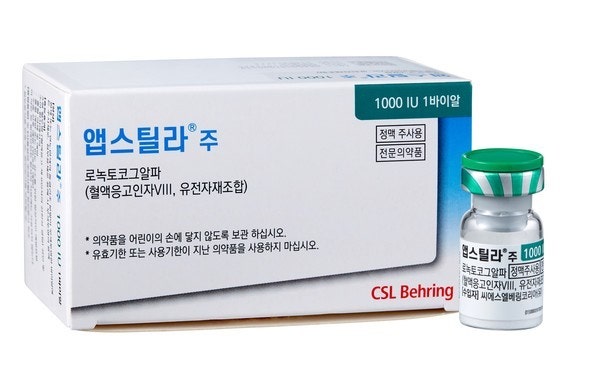
- SK chemicals' Afstyla will be removed from the reimb list
- by Lee, Tak-Sun Aug 25, 2025 06:08am
- Product photo of Afstyla SK chemicals' hemophilia A treatment, Afstyla, which was out-licensed by the company, will be removed from the reimbursement list due to a lack of record of supply performance. The product's delisting was previously deferred in 2023 through conditional negotiation, but with no sales to show since, the decision to remove it has become final. This is a regrettable outcome for a product developed by a domestic company and out-licensed globally. According to industry sources, Afstyla will be removed from the reimbursement list, effective September 1. The product received a decision for removal in 2023 after a review of non-billed drugs revealed no sales over the previous three years; however, its delisting was put on hold through conditional negotiation. However, with no sales recorded within the negotiation period, the decision to remove it has been finalized. Afstyla is currently on the reimbursement list with a price cap of KRW 625 per dose. Afstyla is a biosimilar developed by SK chemicals and out-licensed to CSL Behring Australia during the clinical trial phase in 2009. CSL Behring completed the trials and received approval for the drug in the U.S. and Europe. In 2020, it obtained domestic approval and was added to the reimbursement list in 2021. SK Plasma, an affiliate of SK chemicals, was responsible for domestic sales. In 2020, it obtained domestic approval in Korea and was added to the reimbursement list in 2021. SK Plasma, an affiliate of SK chemicals, was responsible for domestic sales. However, entering the hemophilia market in Korea proved difficult due to the strong presence of established pharmaceutical companies, such as Green Cross, which has a solid position with the hemophilia market. It is also believed that the market outlook was not bright due to the successive launch of competing new drugs. In a 2023 review of non-billed drugs, the product had no production track record for the past three years. However, the manufacturer was able to delay delisting by explaining that sales would soon occur or production would be completed. But after two years with no sales, Afstyla will be removed from the reimbursement list. CSL Behring launched Idelvion, a hemophilia B treatment with improved patient convenience, in Korea last year. With the delisting of Afstyla, it appears CSL Behring will shift its focus to supplying Idelvion.
- Policy
- NHIU ‘Eradicate rebates with INN-based prescriptions'
- by Lee, Tak-Sun Aug 25, 2025 06:06am
- The National Health Insurance Trade Union stressed the need to eradicate pharmaceutical rebates by improving the drug pricing system and distribution structure to those of advanced country standards. The union also advocated the introduction of generic substitution through international nonproprietary name prescriptions. On the 18th, it was revealed through a prosecution investigation that a pharmaceutical wholesaler had provided approximately KRW 50 billion in illegal rebates to three general hospitals through a new scheme involving the establishment of a shell company to pay dividends. The NHIU stated, “The inflated drug costs resulting from illegal rebates and bid-rigging are being passed on to the public in the form of higher medical expenses, leading to unnecessary overprescription of medications and causing serious harm to the health and lives of citizens. This serves as a major factor contributing to the leakage of Koera’s health insurance finances, ultimately resulting in increased health insurance premiums for both citizens and businesses.” The provision of rebates by medical professionals is strictly prohibited under the current Medical Services Act (Article 23-5) and the Pharmaceutical Affairs Act (Article 47). Despite this, in July, the Korea National Police Agency's National Investigation Headquarters announced that it had identified 597 individuals in the medical and pharmaceutical fields suspected of illegal rebates through a special crackdown on illegal rebates and public official corruption. This investigation result demonstrated that illegal rebates in the medical field remain widespread despite the introduction of the dual punishment system for rebates 15 years ago. According to the NHIU, recent illegal rebates in the medical field have been cleverly evading enforcement by exploiting investigative blind spots caused by limited manpower in law enforcement agencies such as the police and prosecution, through more indirect and sophisticated methods such as academic support and consulting. In some cases, an “indirect supplier” is involved as an additional distribution channel between wholesalers and medical institutions, and to circumvent the principle of “one person, one facility” under the Medical Services Act (prohibiting duplicate establishments), wholsalers have been registering indirect suppliers under the names of family members or others related to the medical professionals, leveraging their monopolistic position to extort unfairly low prices from pharmaceutical suppliers and provide illegal rebates to medical professionals. Recently, the Organization for Economic Cooperation and Development (OECD) reported in its ‘Health Statistics 2025’ that South Korea's pharmaceutical expenses are 47% higher than the average of OECD member countries. The NHIU stated, “Enforcement and punishment alone are insufficient to break the vicious cycle of illegal pharmaceutical rebates that recur every year. Fundamental solutions include improving drug pricing systems and distribution structures through price competition among suppliers, such as government tenders and individual drug price negotiations, or introducing flexible pricing systems like reference pricing. Also, the activation of generic substitution based on product names or ingredient names, which is implemented or recommended practice in most advanced countries with separation of prescribing and dispensing systems, is necessary.” They cited Spain as an example. Spain, which has a similar population to South Korea, saves EUR 200 million annually (as of 2017, with a generic substitution rate of 53%) through generic substitution using the International Nonproprietary Name (INN). The union stated that introducing generic substitution through INN-based prescriptions in South Korea could result in annual savings of at least KRW 500 billion in health insurance funds. The NHIU declared, “To eliminate pharmaceutical rebates that threaten the health insurance budget, we will form a united front with labor and civil society organizations that share our vision and set up a proactive and systematic management strategy to improve the drug pricing system and pharmaceutical distribution structure.” It added, “The government and National Assembly must actively pursue legal and institutional reforms to eradicate illegal pharmaceutical rebates. This is because the ‘black money’ from illegal pharmaceutical rebates should no longer be passed on to the public, who already pay the world's highest pharmaceutical costs.” The statement emphasized that pharmaceutical rebates, which exacerbate the public's medical expenses and hinder the sustainable development of the health insurance system, must be eradicated through reforms to establish a drug pricing system and distribution structure on par with those of advanced countries.
- Policy
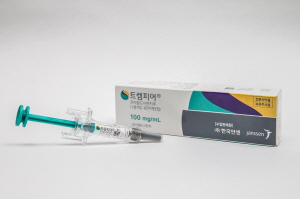
- IV formulation of Korea’s first IL-23 drug Tremfya approved
- by Lee, Hye-Kyung Aug 25, 2025 06:05am
- The intravenous injection formulation of Tremfya, the first interleukin-23 (IL-23) inhibitor in Korea, was recently granted approval, paving the way for the supply of all Tremfya injection formulations in Korea. On the 21st, the Ministry of Food and Drug Safety approved Janssen Korea's Tremfya Intravenous Injection (guselkumab, recombinant DNA). In addition to receiving approval for ‘Tremfya Prefilled Syringe’ in 2018 and ‘Tremfya OnePress Autoinjector’ in 2021, the company has now received approval for the intravenous injection formulation and may now introduce the formulation to Korea. The indications for Tremfya vary depending on the formulation. The pre-filled syringe formulation is indicated for plaque psoriasis, palmoplantar pustulosis, psoriatic arthritis, ulcerative colitis, and Crohn's disease. The intravenous injection recently approved is used for the induction therapy of ulcerative colitis and Crohn's disease. The 200mg intravenous injection is administered intravenously over a minimum of 1 hour on weeks 0, 4, and 8, followed by maintenance therapy using Tremfya Prefilled Syringe Injection or Tremfya OnePress Autoinjector. Therefore, the intravenous injection is indicated only for the treatment of moderate-to-severe active ulcerative colitis or Crohn's disease in adults who have not responded adequately, have lost response, or have intolerance to conventional therapies, including biological agents or small-molecule drugs. Meanwhile, Tremfya is the first and only interleukin-23 inhibitor approved in Korea for psoriatic arthritis, and its reimbursement criteria are being continuously expanded. Psoriatic arthritis is a chronic progressive immune disease characterized by joint inflammation, enthesitis (inflammation at the sites where bones, tendons, and ligaments meet), dactylitis (severe inflammation of the fingers and toes), and pain in the hands and feet, commonly occurring in individuals aged 30–50 years. To date, there is no cure for psoriatic arthritis, and despite available treatment options, many patients experience symptoms that impair their ability to perform daily activities. It is estimated that approximately 9% of psoriasis patients in South Korea develop psoriatic arthritis.
- Policy
- Blockbuster drugs, Atozet·Rosuzet, get price cuts
- by Lee, Tak-Sun Aug 22, 2025 06:07am
- It has been reported that prices for blockbuster drugs, including the hyperlipidemia combination therapies Atozet (Organon) and Rosuzet (Hanmi Pharmaceutical), are expected to be reduced due to increased usage. These hyperlipidemia combination therapies are frequently subject to annual volume-based drug price negotiations (PVA). Additionally, Celltrion's antibody biosimilar Remsima and Dong-A ST's growth hormone product Growtropin-II Inj are also reported to be on the list for price cuts. On August 21, industry sources reported that reimbursement caps for several products would be adjusted as of September 1, under the 'Type-Da' Price-Volume Agreement. The number of blockbuster products is reported to be included this time. The reimbursement cap for Organon's hyperlipidemia combination therapy, Atozet Tab (atorvastatin-ezetimibe), is expected to be reduced by 3.4% following negotiations. The prices of the 10/10mg, 10/20 mg, 10/40 mg, and 10/80 mg products will be adjusted as follows: 10/10 mg from KRW 951 to KRW 918, 10/20mg from KRW 1,209 to KRW 1,168, 10/40mg from KRW 1,299 to KRW 1,255, and 10/80mg from KRW 1,387 to KRW 1,340. Atozet's outpatient prescription sales last year, based on UBIST data, were KRW 118.7 billion, a 16.3% increase from KRW 102.1 billion in the previous year. The increase in prescription sales alone exceeded KRW 16 billion. This year's negotiation targets for Type-Da Price-Volume Agreement include products whose 2024 claim amount increased by more than 60% compared to 2023, or products that increased by more than 10% with the increase exceeding KRW 5 billion. Based on UBIST data, Atozet meets these criteria. Hanmi Pharmaceutical's hyperlipidemia combination therapy, Rosuzet Tab (rosuvastatin-ezetimibe), is also reported to have undergone a price cut. Rosuzet Tab's reimbursement cap is expected to be reduced by 1.3% to 2.1% depending on the dosage. The prices for the 10/10mg dose are expected to be reduced from KRW 1,103 to KRW 1,087, the 10/20mg dose from KRW 1,111 to KRW 1,093, the 10/5mg dose from KRW 789 to KRW 779, and the 10/2.5mg dose from KRW 727 to KRW 712. Rosuzet recorded outpatient prescription sales of KRW 210.2 billion last year, a 17.6% increase from KRW 178.8 billion in the previous year. HK inno.N's Rovazet and Yuhan Corp.'s Rosuvamibe, which contain the same ingredients as Rosuzet, will also have their reimbursement caps lowered in this negotiation. Rovazet recorded outpatient prescription sales of KRW 47.4 billion last year (up 23%), and Rosuvamibe recorded KRW 89.1 billion (up 14.6%) based on UBIST data. Celltrion's antibody biosimilar , Remsima, used for the treatment of rheumatoid arthritis and other conditions, will also have its price adjusted due to increased usage. Remsima is a biosimilar of Remicade, a blockbuster product with global sales of over KRW 1 trillion. Its reimbursement cap is scheduled to be lowered by 1.2% through an agreement with the NHIS. Dong-A ST's growth hormone product, Growtropin-II Inj, is also on the rise.Growtropin-II Inj's reimbursement cap was adjusted last year due to the Price-Volume Agreement as well. Additionally, Janssen's ADHD treatment Concerta OROS ER Tab is expected to see a 3.9% reduction in its reimbursement cap. Meanwhile, this year's Price-Volume Agreement price reduction list reportedly does not include any of the choline alfoscerate products for cognitive enhancement. These products, which included six items last year, had been consistently on the PVA list. However, after the decision was made to apply selective reimbursement to choline alfoscerate products in a reimbursement re-evaluation, pharmaceutical companies shifted their business to alternative products, which seems to have halted their growth.
- Policy
- ALS drug Qalsody is approved in Korea with a condition
- by Lee, Hye-Kyung Aug 22, 2025 06:07am
- The ALS treatment ‘Qalsody (tofersen)’ has been approved under the condition that the results of its therapeutic confirmatory clinical trial be submitted later. According to the advisory council’s review results regarding the safety and efficacy of Qalsody, which was released by the Ministry of Food and Drug Safety on the 20th, the council saw consensus on the need to grant conditional approval for Qalsody, given how ALS worsens over time and treatment options are limited. Biogen's Qalsody is a nucleic acid therapy that binds to SOD1 mRNA in patients with ALS caused by mutations in the superoxide dismutase 1 (SOD1) gene, reducing the synthesis of mutated proteins (SOD1). It received domestic approval from the MFDS on the 20th of this month. According to the CPAC meeting results, experts noted that ALS is a highly severe disease that can be fatal without treatment, and that there are no specific medications available for the condition at the present. In particular, Qalsody’s indication is limited to ALS patients with SOD1 gene mutations. While the mechanism of action involves binding to mRNA, entering the nucleus, and inhibiting SOD1 expression, there were opinions that it is difficult to directly evaluate or reflect the actual SOD1 secreted externally. A member of the CPAC stated, “Based on the submitted data, the drug seems to show an effect when NfL correction is applied. In neurodegenerative diseases, biomarkers can sufficiently explain the extent of neural damage, and as there is scientific evidence on their effect, conditional approval was deemed appropriate.” Another member explained, “This disease is a rare and severe condition with no available treatments in the country, and existing approved drugs are primarily used for symptom relief. Given the clinical trial results based on the NfL biomarker and its potential to control the disease, we deemed that the benefits are significant and agree to grant a conditional approval.” However, there was also an opinion that the completion date of the conditional Phase III clinical trial should be considered, as patients with SOD1 mutations account for less than 3% of all ALS patients, which may require a longer patient recruitment period. Experts also suggested that conditional approval is necessary to provide treatment opportunities for domestic patients, as the drug is already in use overseas. In other words, the council saw that the safety and efficacy of the drug are deemed acceptable based on the data submitted by the pharmaceutical company, and conditional approval is needed to allow patients to benefit from the treatment as soon as possible. Meanwhile, Qalsody was approved in Korea through the accelerated approval process, the Global Innovative products Fast-Track (GIFT), as the 31st product.
- Policy
- MFDS's bill on stable supply of essential drugs in review
- by Lee, Jeong-Hwan Aug 20, 2025 06:22am
- A bill to add “medicines that are essential for healthcare and require stable supply” and “medicines with similar therapeutic effects with no alternative treatments” to the list of national essential medicines, thereby resolving the issue of unstable supply of medicines, is likely to pass the Health and Welfare Committee's legislative subcommittee. At the subcommittee meeting held on the morning of the 19th, the subcommittee members and the Ministry of Food and Drug Safety agreed on the intent of the legislation, but there were some disagreements over the specific wording that should be used to codify the definition, so the subcommittee agreed to pass the bill after going through the amendment process on the same day. The subcommittee members and the MFDS also agreed to accept a provision to revise the designation method for essential medicines to be designated by the Minister of Health and Welfare and the Minister of Food and Drug Safety after consultation with the National Essential Medicine Stable Supply Council. The bill also includes provisions to include “linking drug distribution information” in the scope of the Comprehensive Drug Management Center's duties and to allow the MFDS director to request the Drug Management Information Center to provide and link drug distribution information. On the morning of the same day, the subcommittee members decided to merge the bills proposed by Representative Sun-min Kim of the People Power Party and Representative Mi-hwa Seo of the Democratic Party of Korea and continue deliberations. However, as further discussions on the bill are scheduled at the afternoon subcommittee meeting, there is a possibility that it could pass the subcommittee on the same day if an agreement is reached between the government and the subcommittee members. The bill expands and revises the definition and scope of essential medicines and establishes a legal basis for managing distribution information to contribute to the stable supply of essential medicines. Expanding the definition of essential medicines to strengthen management of drugs with unstable supply The current law defines essential medicine as “medicines that are essential for health and medical care, such as disease control and radiation disaster prevention, but whose stable supply is difficult to ensure through market functions alone, and which are designated by the Minister of Health and Welfare and the Minister of Food and Drug Safety in consultation with the heads of relevant central administrative agencies.” Rep. Sun-min Kim's bill proposed revising the definition of essential medicines to “drugs that are essential for maintaining the national health system, such as disease control and radiation disaster prevention, or that are essential for health and medical care and require a stable supply, as designated by the Minister of Health and Welfare and the Minister of Food and Drug Safety in consultation with the National Essential Medicine Stable Supply Council.” Representative Mi-hwa Seo's bill proposed amending the definition to “medicines that are essential for health and medical care, such as disease control and radiation disaster prevention, but whose stable supply is difficult to ensure through market functions alone, or drugs for which there are no substitutes with similar therapeutic effects, as designated by the Minister of Health and Welfare and the Minister of Food and Drug Safety after consultation with the National Essential Medicine Stable Supply Council pursuant to Article 83-4, Paragraph 3.” The MFDS agreed to reflect both Kim and Seo’s bills and submitted a proposed amendment to the relevant clause. The MFDS proposed to revise the bill so that essential medicines would be designated by the Minister of Health and Welfare and the Minister of Food and Drug Safety after consultation with the National Essential Medicine Stable Supply Council, and to define essential medicine as drugs that are essential for maintaining the national health system, drugs that are essential for health care but difficult to supply stably through market forces alone, and drugs for which there are no substitutes with similar therapeutic effects. The members of the subcommittee raised the need to define essential medicines in one line rather than listing them individually as proposed in the MFDS amendment, and requested a further amendment. The subcommittee is expected to resume its review of the bill in the afternoon based on the amendment. Distribution information linkage included in the scope of the Integrated Pharmaceutical Management Information Center’s duties The MFDS agreed to add “distribution information linkage” to the scope of the Integrated Pharmaceutical Management Information Center’s duties and to allow the MFDS Minister to request the provision and linkage of distribution information in consultation with the director of the information center. The MFDS also accepted provisions to add “drugs that are not designated as essential medicines but require stable supply due to temporary increases in demand” to the scope of drugs managed by the National Essential Medicine Stable Supply Council and to add monitoring of essential drug supply and demand trends to the duties held by the Minister of Health and Welfare and the Minister of Food and Drug Safety. The MFDS also expressed its opinion regarding the provision to specify the composition of the National Essential Medicine Stable Supply Council in the law. Under the current law, the council is composed of 20 government members, including one chairperson, with the remaining details to be specified in the implementing regulations. Rep. Sun-min Kim’s proposal stipulates that the council shall consist of 30 members, including two chairpersons, with government and private-sector members, and that private-sector members shall constitute a majority. Rep. Mi-hwa Seo's proposal stipulates that the council shall consist of 30 members, including one chairperson, with government and private-sector members. The MFDS proposed a council composition similar to Rep Kim’s proposal, consisting of 30 members, including two chairpersons, but added patient group representatives as recommended by Rep Seo. The subcommittee plans to resume deliberations in the afternoon, as it believes that there has not been sufficient discussion on the details of the bill.