- LOGIN
- MemberShip
- 2025-12-18 05:45:27
- Policy
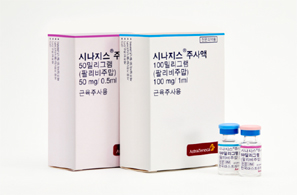
- Shortage of Synagis supply announced…normalized by May
- by Lee, Hye-Kyung Mar 13, 2025 05:57am
- With a shortage in the supply of Synagis (palivizumab), a preventive antibody for respiratory syncytial virus (RSV), which is a main cause of pneumonia and bronchiolitis in infants and young children, expected in Korea, concern arises on how the lack of supply may hinder disease prevention for children in high-risk groups. On the 11th, AstraZeneca Korea notified the Ministry of Food and Drug Safety of a shortage of Synagis 50 mg and 100 mg through a report on supply discontinuation and shortage of drugs. RSV is the most common cause of lower respiratory tract infections in infants and young children, and infants born prematurely, underweight, or with a history of congenital heart disease or bronchopulmonary dysplasia are classified as high-risk groups for RSV infection. Synagis can be administered monthly for 5 months from October to March, during the RSV epidemics season. The first dose should be administered in September before the RSV season begins and should be administered once a month during the RSV season, which lasts until March. The recommended dose is 15 mg per kg of body weight, and the product is available in vials (injections) of 50 mg and 100 mg. AstraZeneca said, “The number of RVS patients has increased more than in previous years, causing a surge in demand for Synagis 50 and 100 mg. Its supply may be temporarily insufficient, due to the current domestic inventory and planned import amounts.” As the hospitalization rate is decreasing as in previous years this March, the current will be of no major concern for patients who have completed receiving the five doses during this RSV epidemic. However, due to the supply shortage in March and April, there is a concern that the preventive effect may be reduced and increase the number of hospitalizations due to RSV infection for the patient group who were unable to receive all the recommended 5 doses. In particular, there is a high possibility that the drug will not be sufficiently effective in preventing the disease in indicated high-risk children. AstraZeneca said, “We will focus on normalizing supply by coordinating the import and domestic release schedules as soon as possible. The expected date for normalization of supply is May 28, 2025.” Meanwhile, Synagis and Sanofi Pasteur Korea's ‘Beyfortus (nirsevimab)' is available in Korea as a preventive vaccine for RSV, but it has different indications. Synagis was approved for the prevention of severe lower respiratory tract disease requiring hospitalization due to RSV in children at high risk of RSV disease, and Beyfortus is indicated for the prevention of lower respiratory tract disease due to RSV. In the case of Synagis, the drug is being administered to high-risk infants and toddlers who are expected to be at high risk of severe RSV disease, such as premature infants.
- Policy
- New types of risk-sharing agreements added for reimbursement
- by Lee, Tak-Sun Mar 12, 2025 05:57am
- The Health Insurance Review and Assessment Service has established two types of risk-sharing schemes, including initial treatment cost reimbursement (Fixed cost refund at initial treatment) and outcome-based reimbursement, to the detailed criteria for drugs subject to negotiation. This appears to reflect the additional content included in the recent revision of the Ministry of Health and Welfare's 'Criteria for the Determination and Adjustment of Drugs.’ According to the industry sources on the 7th, the Health Insurance Review and Assessment Service established two types of risk-sharing agreements in the 'Detailed Evaluation Standards for Medicines subject to Negotiations, Including New Drugs'. The fixed cost refund at the initial treatment type, which was added this time, is a contract in which the applicant refunds a certain percentage of the cost of the first dose used for each patient to the National Health Insurance Service. In addition, an outcome-based refund is a type of contract in which the applicant refunds a certain percentage of the total amount billed to the National Health Insurance Service if the treatment effect is not achieved after tracking and observing the treatment effect of each patient for a certain period of time. As a result, the types of risk-sharing schemes have increased from 4 to 6, including conditional treatment continuation type, mixed-refund type, expenditure cap type, refund type, and patient-specific usage cap type. The post-management entities for the additional types have also been set. The National Health Insurance Service will be responsible for post-management of the initial treatment cost reimbursement type, and the Health Insurance Review and Assessment Service will be responsible for post-management of the outcome-based reimbursement type. The newly established types were approved by the Drug Reimbursement Evaluation Committee a meeting held in February. Meanwhile, the 2 RSA types were added to the recently revised Ministry of Health and Welfare's “Criteria for the Determination and Adjustment of Drugs.” The National Health Insurance Service eased the criteria last year to omit cost-effectiveness evaluation for drugs under the risk-sharing scheme with additional claims of less than KRW 1.5 billion. In addition, the ICER threshold flexibility assessment requirement for innovative drugs was newly established, and the first beneficiary item was the breast cancer drug ‘Trodelvy’ in February. In addition, in December, the NHIS decided to simplify the evaluation for RSA drugs subject to a third round of evaluations, along with a series of measures to ease the industry burden.
- Policy
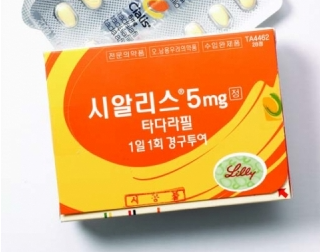
- Tadalafil, only concern for abuse in erectile dysfunction
- by Lee, Hye-Kyung Mar 11, 2025 05:54am
- ’Tadalafil,’ whose indications have been expanded to treat not only erectile dysfunction but also benign prostatic hyperplasia, is expected to partially get rid of the shackles of being a drug with concerns for misuse and abuse. According to the Ministry of Food and Drug Safety's “Partial Amendment to the Regulations on Designation of Drugs of Concern for Misuse and Abuse,” which was announced on the 7th, “tadalafil-containing drugs” that were designated as a drug of concern for misuse and abuse will be revised to “tadalafil-containing drugs for erectile dysfunction.” Tadalafil was designated as a drug of concern for misuse and abuse in 2003 due to concerns over its abuse as a sexual enhancer. In Korea, 3 dosage forms of tadalafil-containing drugs are currently approved (5, 10, and 20 mg) for a total of 187 items, and are indicated for the treatment of erectile dysfunction. However, since 2012, the indication for the low-dose 5 mg has been expanded, breaking the formula that tadalafil is only used for erectile dysfunction. In addition to erectile dysfunction treatment, Lilly Korea has added two indications for Cialis (tadalafil) 5 mg, including ▲benign prostatic hyperplasia and ▲benign prostatic hyperplasia. With the addition of the indications, Cialis 5mg has become a treatment that simultaneously improves the symptoms of erectile dysfunction and benign prostatic hyperplasia, the most common urological diseases in men over the age of 40. Cialis 5mg was the first and only treatment in the world to be approved by the FDA in 2011 for both the treatment of erectile dysfunction and benign prostatic hyperplasia. At the time, Korea was the 5th country to approve the additional indication. Since then, domestic companies have approved a series of generic versions of Cialis 5mg, and there are currently more than 60 low-dose tadalafil products available in the market. In addition, the development of a combination drug containing ingredients such as dapoxetine and tadalafil, following the sildenafil and clomipramine combination, has expanded the treatment options for patients with premature ejaculation and erectile dysfunction. In February, the first combination drug that contains dutasteride and tadalafil, which is used to treat benign prostatic hyperplasia, was approved in Korea. Dongkook Pharmaceutical has received marketing authorization for ‘Uresco Tab,’ an incrementally modified drug for enlarged prostate that contains dutasteride and tadalafil. Benign prostatic hyperplasia is a very common condition where the prostate enlarges with age, causing abnormalities in various urination functions. Drugs that reduce the size of the prostate (finasteride, dutasteride) are used, and various drugs are used in combination to improve symptoms.
- Policy
- Keytruda will be considered for DREC next time
- by Lee, Tak-Sun Mar 11, 2025 05:54am
- Product photo of Keytruda The expanded use of scope application of the immune cancer drug Keytruda, which passed the Cancer Disease Review Committee, is expected to be considered for the Drug Reimbursement Evaluation Committee (DREC) review as soon as April. Previously, the 2nd DREC review for 2025, held on March 6, did not include Keytruda. As Keytruda's DREC review is imminent, the National Health Insurance Service (NHIS) has started to prepare for the next stage. According to industry sources on March 10, the NHIS has initiated a preliminary financial analysis of Keytruda, which is expected to undergo negotiations for an expanded reimbursement scope. Because the approval of the expanded scope of use for Keytruda is expected to cost substantial expenditure, the NHIS may aim to take the lead in negotiations through the preliminary financial analysis. It has been reported that the NHIS will conduct a financial analysis of Keytruda's expanded scope of use through internal meetings. After failing five times, the application of Keytruda for the expanded scope of use passed the Health Insurance Review and Assessment Service (HIRA)'s CDRC on the sixth attempt on the 12th of last month. Of the 17 applications considered for the review, 11 received reimbursement standards. The reimbursement will determined for these 11 indications with reimbursement standards once they pass the DREC and complete negotiations with the NHIS. Keytruda is reimbursed for seven indications in four cancer types, including non-small cell lung cancer, Hodgkin lymphoma, melanoma, and urothelial cancer. Keytruda's yearly claim amount exceeds KRW 400 billion. Therefore, if 11 indications are additionally reimbursed, it will substantially cost the National Health Insurance finance. Because of this, the key to the expanded scope of use negotiations will be the extent to which the pharmaceutical company will take a share of the finance. Consequently, the NHIS reportedly initiated preliminary analysis before negotiating even though the drug had not passed the DREC. The completion of the DREC review is expected in early April. Industry personnel said, "Although the drug was not considered for the DREC in March, it passed the CDRC in February, so there is a chance that it will be considered for the DREC soon," adding, "Because the CDRC carefully considered this item up to six times, the drug will likely to pass the DREC quickly." A drug under the Risk Sharing Agreement (RSA) with a claim amount over KRW 1.5 billion has to pass the DREC review to receive an expanded scope of use. The review outcome will be announced on that day.
- Policy
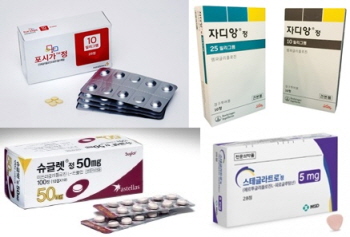
- 734 SGLT2is add precautions for ketoacidosis
- by Lee, Hye-Kyung Mar 10, 2025 05:50am
- SGLT-2 inhibitors approved in Korea Precautions for antidiabetic SGLT2 inhibitors will be strengthened in the near future. 734 ertugliflozin, empagliflozin, and dapagliflozin drugs that have been approved in Korea are expected to be subject to the changed regulations. The Ministry of Food and Drug Safety will prepare an ‘Order for labeling changes (draft)’ based on the safety information of SGLT2 inhibitors by the 20th of this month and conduct an opinion inquiry thereafter. This draft order for changes was made based on the 'Results of the review of safety information on SGLT2 inhibitor-related drugs' by Health Canada (HC) and the US Food and Drug Administration (FDA). Representative products include AstraZeneca Korea's ‘Sidapvia Tab (dapagliflozin, sitagliptin)', HK Inno. N’s ‘DapaN Tab (dapagliflozin propanediol hydrate)', Boehringer Ingelheim Korea’s ‘Jardiance Tab (empagliflozin)', and MSD Korea’s ' Steglatro Tab (ertugliflozin L-pyroglutamic acid).’ The labeling changes for the SGLT2 inhibitor class will be made in the 'General Precautions' section. In all products that have been announced, the 'ketogenic diet' will be added as a factor that is likely to cause ketoacidosis. In addition, pancreatic disorders that cause insulin deficiency will include not only type 1 but also type 2 diabetes. Previously, risk factors for ketoacidosis included reduced insulin doses, acute febrile illness, calorie intake restriction due to illness or surgery, and alcohol abuse. The precautions for combination drugs such as dapagliflozin-sitagliptin, dapagliflozin-metformin, dapagliflozin-metformin-sitagliptin, dapagliflozin-glimepiride, dapagliflozin-pioglitazone, dapagliflozin-linagliptin, dapagliflozin-saxagliptin, dapagliflozin-evogliptin, dapagliflozin-evogliptin-metformin, and dapagliflozin-gemigliptin will become slightly stricter. The changes include the addition of a precaution about ketoacidosis: “Diabetes and ketoacidosis may persist longer than the expected duration and urine glucose excretion may persist for 3 days after discontinuation of administration. However, there have been post-marketing reports of diabetes and ketoacidosis persisting beyond 6 days and up to 2 weeks after discontinuation of SGLT2 inhibitor administration.” The Ministry of Food and Drug Safety has asked associations to notify their members of the changes so that review opinions can be submitted.
- Policy
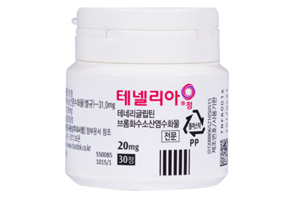
- Handok expands its Tenelia combo lineup
- by Lee, Hye-Kyung Mar 07, 2025 05:56am
- Handok is speeding up the development of a combination drug that combines DPP-4 inhibitor diabetes treatment Tenelia (teneligliptin hydrobromide hydrate)' with SGLT-2 inhibitor 'Jadiance (Empagliflozin). On the 4th, the Ministry of Food and Drug Safety approved an open-label, randomized, empty stomach, single orally administered, two-arm, crossover, Phase I clinical trial to compare and evaluate the safety and pharmacokinetic characteristics of HD-P023 and Tenelia 20 mg and Jardiance 25 mg combination in healthy adults. In 2020, Handok conducted a clinical trial on a triple combination therapy of metformin and empagliflozin and Tenelia, and since last year, it has expanded its development scope to investigate a two-drug combination drug that contains Tenelia and empagliflozin. This is the third time that HD-P023 was approved for a Phase I trial, following approvals in January and June last year. Handok is working on the development of a combination drug for Tenelia, using different doses in each clinical trial. Handok is believed to be making such efforts due to the fact that a large number of generics have been approved upon Tenelia’s patent expiry in October 2022. Domestic companies successfully avoided patent infringement through salt modifications before the expiration of Tenelia’s patent, and forewarned of their entry into the generic market. Currently, 44 items have been approved as generic versions of Tenelia. The patent for the combination drug ‘Tenelia M,' which is a combination of Tenelia and metformin, expired at the same time as that of Tenelia, and the number of approved generic drugs for Tenelia M exceeds 74. As market competition became inevitable with the approval of generics for both the single drug Tenelia and the combination drug Tenelia M, it appears that the company has embarked on the development of a combination drug that can be used with reimbursement with SGLT-2 inhibitors. In particular, since the reimbursement standards for SGLT-2 inhibitor class drugs improved in April 2023, allowing reimbursement of triple-drug therapies such as 'metformin + SGLT-2 + DPP-4' and 'metformin + SGLT-2 + TZD,’ there has been a continuous push for the reimbursement of for two-drug combination therapies as well. According to data Rep Mi-hwa Seo, a member of the Democratic Party of Korea, received from the Ministry of Health and Welfare in January this year, the Ministry of Health and Welfare said it would prepare a plan to improve the reimbursement standards for the two-drug therapy that combine SGLT-2 inhibitor diabetes drugs and DPP-4 inhibitor diabetes drugs. The Ministry of Health and Welfare has finished the Ministry of Food and Drug Safety’s inquiry regarding the request from the Korean Diabetes Association, collected expert opinions from related societies and others, and has stated that it is in discussions with the KDA to prepare additional supplementary data to improve the reimbursement standard. The Ministry of Health and Welfare =, “We will also review whether it is possible to reimburse the combination of SGLT-2 inhibitors and diabetes drugs as one of the two-drug therapies, taking into account the Ministry of Food and Drug Safety’s approval status and the opinions of experts. However, as reimbursement expansion of the diabetes drugs has a significant impact on the finances of the National Health Insurance, it will have to be implemented step by step after ample discussion.” Meanwhile, according to the pharmaceutical market research institution UBIST, the amount of outpatient prescriptions for Tenelia last year was KRW 24.46541 billion and KRW 27.21524 billion for Tenelia M.
- Policy
- CKD and Daewoong Bio enter donepezil-memantine combo market
- by Lee, Tak-Sun Mar 06, 2025 05:55am
- Chong Kun Dang Pharmaceutical and Daewoong Bio will enter the donepezil-memantine combination drug market that was launched this month. Although Chong Kun Dang and Daewoong Bio’s products were not included in the initial list of approved products, the companies will join the market in the form of a license transfer and co-promotion agreement. As both companies own the No. 1 and No. 2 products choline alfoscerate products in Korea -Chong Kun Dang Gliatirin and Gliatamin – which are brain function enhancers, their entry into the donepezil-memantine combination market is expected to increase the scale of product performance more than expected. According to industry sources on the 5th, Chong Kun Dang received permission from Korea Arlico Pharm to transfer the license for its donepezil-memantine combination drug and registered it with the Ministry of Food and Drug Safety in January. Chong Kun Dang Neuropezil M 10/20mg is the main product. The transfer of the license delayed the reimbursement listing process for the drug, which resulted in a later launch date than other pharmaceutical companies. This month, 7 donepezil-memantine combination drugs - Hyundai Pharm’s DM Duo Tab, Yungjin Pharm’s Demenduo Tab, Bukwang Pharm’s Ariplus Tab, Ildong Pharm’s Memansept Tab, Whanin Pharm’s Domentia Tab, Hutecs Korea Pharmaceutical’s Altsucomp Tab, Korean Drug’s Neurocept Duo Tab – were released to the market. Chong Kun Dang plans to release its product in April. Daewoong Bio will start selling Demenduo through a copromotion agreement with Yungjin Pharm starting this month. The donepezil-memantine combination was initially approved in the United States in 2014 and was then approved in Spain, Greece, and Croatia since, but this is the first time the combination was approved in Korea. The domestic patent holder is Hyundai Pharm, which has demonstrated equivalence and toxicity safety through comparative tests of donepezil and memantine administered alone and in combination. In Korea, it is used as an alternative to donepezil and memantine combination therapy for the treatment of moderate-to-severe Alzheimer's disease. The entry of Chong Kun Dang and Daewoong Bio in this dementia combination drug market is expected to heat sales promotion activities. The two companies have an established nationwide distribution network for geriatric drugs through their choline alfoscerate-based drugs. Last year, according to UBIST, Chong Kun Dang’s Gliatirin recorded KRW 121.3 billion in outpatient prescription sales, while Daewoong Bio’s Gliatamin recorded KRW 159.7 billion, continuing their popularity. However, choline alfoscerate’s reimbursement standard was reduced through a re-evaluation, and the Ministry of Food and Drug Safety is also conducting a clinical re-evaluation on its efficacy, so both companies had to look for products that can replace choline alfoscerate. Although the use of this dementia combination drug and the brain function enhancer choline alfoscerate are different, they are mostly used by the elderly, so it is analyzed that if the companies utilize their established distributor base, this may increase the companies’ short-term performance. Donepezil and memantine are the most widely used ingredients in the dementia drug market. Donepezil's original drug Aricept recorded KRW 95.8 billion in outpatient prescriptions last year, while memantine's original drug Ebixa recorded KRW 19 billion, with the combined prescription value of the two drugs exceeding KRW 100 billion. However, some analysts say that their expected market size is limited because the prescription rate of the combination of the two ingredients is smaller than that of each single ingredient. Interest is gathering as to whether the new dementia combination drug market, which has been joined by Chong Kun Dang and Daewoong Bio, which are strong players in the geriatric drug market, will see success in Korea.
- Policy
- Bill for vaccinating 'shingles for elderly·HPV for males'
- by Lee, Jeong-Hwan Mar 05, 2025 06:00am
- The Democratic Party of Korea Rep. issues a bill The Democratic Party of Korea is pursuing changes to the law to expand the free-of-charge vaccination program, requiring the government to cover the costs of shingles vaccines for adults 65 and above and human papillomavirus (HPV) vaccination for males below 17 years. Given that the Yoon Suk Yeol government's pledges during the candidacy to expand the National Immunization Program (NIP) to include shingles and HPV vaccines fell through, the National Assembly aims to implement this with the legislation to protect national health. On March 4, the Democratic Party of Korea Rep. Park Heeseong announced the bill to make partial revision to the 'Infectious Disease Control and Prevention Act.' Rep. Park Heeseong pointed out that the government made a candidacy pledge to provide shingles vaccinations for adults aged 65 and above and HPV vaccines for boys aged 12 and above. However, it was not included in the government budget, suggesting that the pledge has fallen through. Rep. Park Heeseong said that shingles vaccinations have high preventative effects, but since they are still non-reimbursed, this poses a substantial financial burden for individuals facing cost differences. Rep. Park Heeseong highlighted that HIP infection is part of the NIP, but the law limits the range of free-of-cost vaccination to females aged 12 or between 12 and 26. Rep. Park Heeseong believes that the government is not implementing vaccination despite proven evidence, including cost-effectiveness and disease burden, for introducing single vaccinations for older people free-of-cost and HPV vaccinations for males at no cost. Therefore, Rep. Park Heeseong drafted a bill to add shingles vaccination to the NIP and expand the HPV vaccination NIP to include males. The bill newly added·established Clause 18 to Article 24 of the NIP to include 'shingles.' The bill amended the current law to include a new 'Article 24-2 on human papillomavirus (HPV) infection vaccination.' The bill's amendment details that the mayor of a special self-governing city, governor of a special self-governing province, or the mayor·governor·head of Gu is now required to administer HPV vaccinations through local public health centers for females aged 26 or younger and males aged 17 or younger, as a measure to prevent HPV infection. Additionally, the bill's amendment to Article 64 (expenses paid by special self-governing provincess, cities, Gun, and Gu) now includes HPV vaccination. This change establishes the basis for local governments to cover vaccination costs, ensuring that patients can receive the vaccine free of charge.
- Policy
- MFDS designates etomidate a psychoactive drug
- by Lee, Hye-Kyung Mar 04, 2025 05:57am
- The Ministry of Food and Drug Safety (Minister Yu-Kyoung Oh) announced on February 28 that it will preannounce an amendment to the Enforcement Decree of the Narcotics Control Act that will designate 7 substances, including hexahydrocannabinol, which is scheduled to be designated as a controlled substance by the United Nations, as narcotics or psychotropic substances. The amendment is open for opinion collection until April 10. Five substances (4 narcotics and 1 psychotropic drug) that will be newly designated as narcotics this time are those designated as narcotics or psychotropic drugs at the 68th United Nations Commission on Narcotic Drugs (CND) and two psychotropic drugs including etomidate that have been designated as narcotics by the Narcotics Safety and Risk Management Deliberative Committee. Substances newly designated as narcotics by the United Nations Commission on Narcotic Drugs are new narcotics synthesized by changing the structure of existing narcotics. The Ministry of Food and Drug Safety is quickly identifying international narcotics trends and has already designated and managed some drugs as temporary narcotics. The proposed revision also includes etomidate, a general anesthetic induction agent. Since there were cases of illegal administration or abuse of etomidate in some medical institutions, it will be designated as a narcotic drug in advance and actively managed for their safe use in Korea. The Ministry of Food and Drug Safety said, “We expect that this designation of narcotics will be in harmony with international drug regulations and will actively respond to the problem of domestic drug abuse. We will continue to do our best to safely manage narcotics for the health and safety of the people.”
- Policy
- NA starts Special Act to support the pharma and bio industry
- by Lee, Jeong-Hwan Feb 27, 2025 05:56am
- The National Assembly is pushing for the enactment of a special law to bring together the capabilities at the national level to promote and foster the biopharmaceutical industry. The policy agenda includes the establishment of a basic plan every 5 years, the establishment of a Pharmaceutical Biohealth Innovation Committee, and the provision of policy support such as research and development facilities, tax incentives for bio special zones, and practical support such as substantial budget support and tax cuts. On the 24th, Rep. Il-Young Jung, a member of the National Assembly's Strategy and Finance Committee, and the Democratic Party of Korea, proposed the ‘Special Act on the Promotion of the Pharmaceutical Biohealth Industry and Strengthening of Competence (Bio Special Act).’ As the bio industry is a core industry with strategic value in health, medicine, society, economy, and security, the need for support for biotechnology development and the industry as a whole has been raised. In particular, after the COVID-19 pandemic has passed, the bio industry has established itself as a national strategic industry that has a significant impact not only on the health of the people but also on the national economy and security, and there has been a growing demand to foster the industry at the national level. In response, Rep Il-Young Jung proposed the Bio Special Act to enable the government to provide comprehensive support and foster the bio industry in a timely manner, and establish a legal basis for supporting the bio industry. The bill includes ▲the establishment and implementation of a five-year basic plan, ▲the establishment and operation of the Pharmaceutical Bio-Health Innovation Committee, ▲budget support for R&D and infrastructure construction, ▲designation of bio-special zones, and the ▲provision of special exemptions such as tax support for special zones and exemption from preliminary feasibility studies and regulatory improvements. In addition, the plan includes ▲tax and financial support for foreign-invested companies and companies returning to Korea, ▲the creation of a bio-industry fund, and ▲support for the training of professionals and vocational training. Among these, the tax support is intended to reduce taxes on pharmaceutical and bio-health companies in accordance with the relevant tax laws, such as the Act On Restriction On Special Cases Concerning Taxation and Act On Restriction On Special Cases Concerning Local Taxation, to promote investment in the pharmaceutical and bio-health industry and revitalize special zones. “The current Korean economy is facing a difficult situation with a growth rate of just over 1%, and this economic difficulty is largely due to structural problems in the Korean industrial structure, rather than a temporary phenomenon,” said Rep. Il-Young Jung. He also pointed out that ”The trade environment is deteriorating further due to domestic political instability and the tariff war that began under the Trump administration in the United States, which will significantly hurt the semiconductor and automobile industries, which have driven the Korean economy.” “The need for new growth industries has been continuously raised to overcome the difficulties of the Korean economy, and the bio industry is the most in the spotlight,” said Jung, ”It is necessary to intensively foster the bio industry and quickly develop it into a major industry that will lead the Korean economy.” “The global bio industry market will grow to about USD 3.3 trillion by 2027. We will establish a legal and institutional foundation for the promotion of the bio industry through the Bio Special Act so that the Korean bio industry can become globally competitive and serve as a new economic engine for Korea, to succeed the semiconductors and automobile industry.”