- LOGIN
- MemberShip
- 2025-12-17 11:50:23
- Policy
- Xofluza, Ongentys generics apply for approval
- by Lee, Tak-Sun Dec 03, 2025 08:43am
- Roche’s flu treatment XofluzaAttention is focused on whether market competition will intensify with approval applications being filed for items where no generic drugs have emerged until now.However, given that the original drugs' patents are registered, overcoming patent barriers is expected to be key for entering the market.According to the Ministry of Food and Drug Safety (MFDS) on the 2nd, applications for generic versions of the influenza treatment Xofluza Tab (baloxavir marboxil, Roche) and the Parkinson's disease treatment Ongentys Cap (opicapone, SK Chemicals) have recently been submitted for approval.MFDS has notified the originator companies of the applications in accordance with Korea’s patent–approval linkage system, since the patents for these originators are registered on the Green List.This gives the originators the right to seek sales injunctions on grounds of patent infringement.Xofluza is Roche's next-generation influenza antiviral, an upgrade from Tamiflu. While Tamiflu requires a 5-day course, Xofluza offers the advantage of treatment and prevention with just a single dose, providing much improvement in terms of dosing convenience.However, since receiving domestic marketing approval in November 2019, Xofluza has not yet been listed for reimbursement and therefore is not being sold properly in the market.Meanwhile, follow-on manufacturers are eyeing the market with generics containing the same active ingredient. Last month, Kwangdong Pharmaceutical filed a passive scope confirmation trial to circumvent Xofluza’s formulation patent (a solid formulation having excellent stability).This marks the first detected application filing following the patent challenge. Should Kwangdong Pharmaceutical succeed in circumventing the formulation patent, an early launch of generics will become possible.Ongentys, which was approved the same month as Xofluza, has also been targeted by generic companies. The drug is used as adjunct therapy for Parkinson’s disease patients experiencing motor fluctuations who do not adequately respond to standard levodopa/dopa-decarboxylase inhibitor (DDCI) regimens.Ongentys was added to the reimbursement list in October last year at KRW 2,515 per capsule. Development of a generic version began less than a year after its reimbursement listing.Myung In Pharm filed a passive scope confirmation trial in May to challenge Ongentys’ composition patent (pharmaceutical composition containing a nitrocatechol derivative and its manufacturing processes).Applications filed for generic versions were detected soon after, accelerating the opening of the follow-on generic market.The market entry of generics for Xofluza and Ongentys hinges on their patents. For Xofluza, 2 substance patents are scheduled to expire in 2031 and 2036, respectively.Even if companies succeed in avoiding the formulation patent expiring in 2039 through patent challenges, the substance patents are expected to delay generic market entry by another 10 years. Therefore, analysis suggests that overcoming the substance patent barrier is necessary for generics to enter the market sooner and start sales in earnest.For Ongentys Cap, the substance patent expires in July 2026 and the use patent in October 2027. Therefore, if the composition patent expiring in 2030 is successfully avoided, a generic launch could occur within 2 years.However, it remains uncertain whether the Patent Trial and Appeal Board will rule in favor of the generic challengers.Meanwhile, another application has been filed for the generic version of Hanmi Pharmaceutical’s AmosartanQ, for which Huons earlier became the first to receive generic approval in September.Huons obtained approval for BesylsartanQ Tab, which contains an “alternative salt form” product containing the same active ingredients (amlodipine camsylate+ losartan + rosuvastatin). However, the formulation patent for AmosartanQ is expected to remain in force until November 2033, making its market launch uncertain. Huons has filed a passive claim scope confirmation trial against this patent, aiming to circumvent it.Amid this situation, another pharmaceutical company has now applied for approval of a generic version.
- Policy
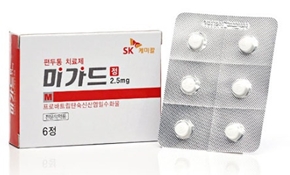
- First Migard generic listed in KOR
- by Jung, Heung-Jun Dec 02, 2025 12:37pm
- The first generic version of Migard will be listed for reimbursement in Korea next month.Frotriptan Tab, Myung In Pharm’s first generic version of SK Chemicals' Migard Tab, will be listed for reimbursement next month, marking the beginning of full-scale competition.With this listing, Myung In Pharm strengthened its position in the migraine market, alongside its existing products such as Sumatran (sumatriptan succinate) and Topamate (topiramate).According to industry sources on the 28th, Myung In Pharm’s Frotriptan Tab 2.5 mg (frovatriptan succinate monohydrate) will be reimbursed starting next month. As the first generic, it receives a 59.5% premium, with the upper reimbursement limit set at KRW 2,038.Last year, Myung In Pharm became the first domestic company to conduct a bioequivalence study for the launch of a generic version of Migard. It obtained the first generic approval this June.Migard is a migraine treatment containing frovatriptan that received domestic approval in 2009. It is used for the acute treatment of migraine with or without aura.According to the market research firm UBIST, Migard recorded KRW 2.5 billion in sales last year, representing a 16% increase from the previous year.With the entry of generics, SK Chemicals and Myungin Pharmaceutical will now compete in the previously uncontested misgraine treatment market.While Migard’s standalone sales may not suggest a large market, the broader triptan-class market, including agents such as sumatriptan, is not small. The acute migraine treatment market is estimated to be worth approximately KRW 23 billion.Several pharmaceutical companies, including Yuyu Pharma, Daewoong Bio, Han Wha Pharma, and Reyon Pharmaceutical, already market products containing sumatriptan, naratriptan, and other triptan-class ingredients.Myung In Pharm, known for its strong CNS portfolio, continues to expand its migraine lineup, adding Frotriptan to its existing line of products such as Sumatran (sumatriptan succinate), Topamate (topiramate), and Poxen (naproxen sodium).SK Chemicals, its direct competitor, has also been strengthening its migraine portfolio. In addition to Migard, it launched Suvexx, a combination of sumatriptan and the anti-inflammatory agent naproxen, last year. Also, in October last year, it further expanded its lineup by acquiring the original zolmitriptan product Zomig Tab from AstraZeneca.
- Policy
- Biannual post-listing price cuts to be made
- by Jung, Heung-Jun Dec 02, 2025 12:35pm
- Price reductions under the post-listing management system, including price-volume agreements, will be consolidated into twice-yearly adjustments, significantly reducing uncertainty over price fluctuations.However, with the reduced frequency, large-scale price adjustments in both the first and second halves of the year have become inevitable.While the practice of lowering drug prices after actual transaction price investigations—a longstanding industry grievance—will be abolished, the incentive for low-price purchases will increase 2.5-fold, potentially triggering cutthroat bidding competition in large hospital tenders.The Ministry of Health and Welfare (MoHW) announced its drug-pricing reform plan on the 28th and will collect opinions through Q1 next year before implementing changes in July. Dailypharm reviewed how the reform, especially the post-listing management restructuring, will affect the field.Post-management overhaul: Drug price cuts in April & October... will reduce uncertainty but bring larger-scale adjustmentsFrequent drug price reductions under various post-management systems caused confusion in the field.Going forward, the timing for post-management price adjustments will be fixed annually in April and October, making it easier for the field to prepare for price changes.Even within the PVA system, reductions previously occurred at different times depending on the category (A/B/C), leading to complaints about constant adjustments. Price cuts driven by increased utilization after indication expansion or reimbursement expansion were also applied on a rolling basis throughout the year.Starting in 2027, the consolidation to twice-yearly adjustments is expected to eliminate these complaints. However, since drug price adjustments will be reduced to once per half-year (first and second half), large-scale reductions will be unavoidable each time.From the pharmacy's perspective, while the confusion caused by the unpredictable timing of price reductions will be resolved, the workload is expected to increase with adjustments occurring only once per half-year.The actual transaction price cut will be eliminated because the government views the administrative burden as excessive relative to the benefit. Instead, the incentive for low-price purchasing will increase from 20% to 50%, with the intent of inducing price competition that naturally lowers transaction prices.While the industry welcomes the abolition of actual transaction price reductions, it expresses concern over the incentive expansion policy. This is because, considering the de facto dominant-subordinate relationship between large hospitals and pharmaceutical/distribution companies, adverse effects are anticipated.If the incentives received by medical institutions increase by approximately 2.5 times, the demand for lower prices will grow, and the industry fears an increase in cutthroat competition where losses must be absorbed.The industry's primary demand is for supplementary measures, such as minimum price floors and eligibility review systems.Preemptive measures for 2012 adjusted drug price reductions... Companies with post-2013 listings express anxietyThe government will take preemptive action on products that have maintained the 53.55% price level for 13 years since the 2012 blanket reduction.If the reform is finalized as is, HIRA will select eligible products among the 6,500 that were reduced in 2012, review them, and begin applying new price cuts.The following are excluded from the price reduction targets: ▲ Drugs receiving premium pricing ▲Exit-prevention drugs, low-cost drugs, and orphan drugs ▲ Drugs listed exclusively ▲ Drugs whose prices have increased in the last 5 years due to supply instability ▲ Basic infusion solutions and radiopharmaceuticals ▲ Oxygen and nitrous oxide.Pharmaceutical companies holding items listed since 2013 are also excluded from immediate measures, but they worry the government might expand the scope of price reductions.Furthermore, in the second half of next year, the uniform surcharge for generic drug listings will be abolished, replaced by a differential surcharge based on innovation and contribution to supply stability. As the first half of the year represents the last opportunity for listing, applications are expected to flood in during the first and second quarters.Moving forward, the R&D-to-sales ratio will become crucial for receiving drug price premiums. Differential premiums will be applied based on criteria such as the top 30% and bottom 70% of innovative pharmaceutical companies. Companies hovering ambiguously near the cutoff line are likely to engage in a game of brinkmanship over the extent of their R&D investment expansion.As no absolute numerical threshold is set, the government appears to anticipate a competitive surge in R&D investment.Stepped price reductions for generics will also be strengthened. Currently, the price of the 21st generic is set at 85% of the lowest price. This will be applied starting from the 11th generic. The intent is to reduce prices by 5% each step from the first generic's calculated price to prevent indiscriminate proliferation of generics.This reform is particularly painful for small and medium-sized pharmaceutical companies, as the government's clear goal is to induce new drug development through strict generic drug price management and to transform the industry ecosystem, which is overly reliant on copy drugs.If companies conclude that obtaining add-on pricing will be difficult and that the benefit of listing is reduced, they may shift more aggressively toward non-reimbursed products.
- Policy
- Gvn’t ‘No blanket drug price cuts will be made’
- by Lee, Jeong-Hwan Dec 02, 2025 12:34pm
- Yeon-sook Kim (Director of the Pharmaceutical Benefits Division), Joong-kyu Lee (Director-General for Health Insurance Policy), and Gi-heon Bae (Deputy Director, Pharmaceutical Benefits Division) from MOHW explained the details and intent of the drug price reform plan. "During the blanket drug price reduction, prices of approximately 6,000 items were cut. Since then, 13 years have passed, and 4,500 items have either not been reduced at all or only reduced to 45% from 53.55%. Starting next year, the price reductions will be made over the next 3 years to these 4,500 items. There is little room for debate about price cuts over these listed generics since they've been generating excessive profits for over a decade. The remaining 15,000+ already-listed generics are not targets for this price reduction. We plan to review the other listed generics only after completing the adjustment process for the initial 4,500 items. ‘Reviewing’ does not mean we intend to cut their prices."As the government unveiled a plan to lower the generic price rate from 53.55% to the 40% range of the original drug’s price, it confirmed that prices of 4,500 pre-listed generics will be cut first when the reform is implemented in the second half of next year (July). The expected savings in National Health Insurance drug expenditure from this measure amount to KRW 1 trillion.Based on the 6,000 items subject to the 53.55% blanket generic price reduction applied in 2012, the plan targets 3,000 items whose prices have barely decreased (maintaining 53.55%~50%) since then up to the present (2025), and 1,500 items that saw only a slight decrease (maintaining 50% to 45% range). The plan is to reduce prices to the 40% range over 3 years, targeting only these items.For the 3,000 generics maintaining prices between 53.55% and 50%, adjustments will begin next year (2026) and will be reduced to the 40% range by 2028. The 1,500 generics in the 50% to 45% range will begin adjustments the year after next (2027) and be reduced to the 40% range by 2029.The government has clearly stated that it will not reduce the prices of generics listed from April 2012, when the blanket price reduction for generics began, to July next year, when the reform plan takes effect, at least until the price adjustments for 4,500 items are completed.However, starting in 2030, when the price reduction process for the 4,500 items concludes, the government intends to establish a mechanism to periodically review generics listed between April 2012 and June 2026 to assess the need for price adjustments.On the 30th, Joong-kyu Lee (Director General for Health Insurance Policy), Yeon-sook Kim (Director of the Pharmaceutical Benefits Division), and Gi-heon Bae (Deputy Director, Pharmaceutical Benefits Division) held a briefing with the MOHW press corp to explain the sequential adjustment plan for pre-listed generics.4,500 drugs to see 40% price cuts by 2029... “KRW 1 trillion in drug cost savings”The Ministry expects that reducing the prices of 4,500 existing generic drugs by around 40% over the next 3-4 years, starting next year, will yield savings of approximately KRW 1 trillion in National Health Insurance finances.Lee explained, “These 4,500 generics have kept their prices unchanged for more than 10 years since 2012 and have enjoyed excessive profits. The need for reduction is hardly disputable.”Simply put, since this generic drug price reduction targets only 4,500 items out of the 6,000 that underwent a blanket price reduction in 2012, it is unlikely to provoke significant backlash from the domestic pharmaceutical industry. It's essentially saying, ‘These generics deserve the cut.’But the Korean pharmaceutical industry strongly disagrees.They argue that maintaining the 53.55% price level required real investment efforts, such as conducting their own bioequivalence tests and registering DMFs (Drug Master Files), and meeting various regulatory criteria. Therefore, criticizing the price as excessive profit and cutting it simply because it wasn't reduced amounts to the Ministry of Health and Welfare itself, negating the tiered pricing system it has set standards and requirements for.Moreover, while the price reduction mechanism for multinational pharmaceutical companies focused on original products converges to zero, the Ministry announced it would accept systems favored by multinationals—such as the flexible pricing contract system that allows different listed and actual transaction prices, and the differential pricing system by indication. This has led to cynical reactions within the pharmaceutical industry, including “a reform plan that cuts domestic generic drug prices while further bolstering prices for imported originals.”Lee Jung-kyu: “The focus of the reform plan is on rewarding innovative R&D, not restructuring generics.”Director-General Lee emphasized that the reform plan's significance lies in encouraging innovative new drug R&D (research and development) rather than restructuring domestic pharmaceutical companies or domestic generics. He stressed that it aims to overhaul the drug pricing system into one that “favors as hard as possible” the drug prices for companies developing and producing medicines with unstable supply, namely essential medicines and market exit prevention drugs.He specifically clarified that at the current phase, the government is not considering price reduction mechanisms for generics listed between April 2012 and June 2026. This does not mean the prices of these existing generics won't be cut, but rather that after reducing prices for the initial 4,500 drugs, the necessity for further reductions will be discussed and considered with the pharmaceutical industry.The implication is that plans for handling the prices of these existing generics, which were not touched this time, will be determined gradually going forward.Domestic pharmaceutical companies maintain that retroactively applying the 40% generic drug price reduction reform to listed generics is unreasonable. Should the Ministry of Health and Welfare proceed with adjusting prices for generics listed since 2012, industry backlash is expected to be even stronger than it is now.Lee stated, “Currently, generics approved in 2012 are the target for price reductions. The 15,000 generics listed afterward will be reviewed later. This does not mean we intend to reduce prices periodically. It means we will thoroughly review the status of all generics listed in Korea.”He further emphasized, “The fact that 4,500 generics either maintained their prices at the 53.55% level or saw reductions of only up to 45% might warrant an audit from the perspective of the Ministry of Health and Welfare's National Health Insurance Policy Bureau and Pharmaceutical Benefits Division. We will regulate generics that have not seen price reductions for over a decade and have generated excessive profits.”Lee stated, “In 2012, the specific goal was to unilaterally reduce generic drug prices across the board. Yet, the amount of reduction made was not significant. This reform is not about restructuring generics; the policy direction is to properly support pharmaceutical companies excelling in new drug R&D and stable supply. It is an administrative measure to naturally reduce the prices of simple generics and incentivize their elimination from the market.”Preferential pricing for essential drugs, exit-prevention drugs, and domestic raw materials is also being discussed… “Active substitution to relieve public anxiety”To incentivize R&D, the reform links pricing with the “Korea Innovative Pharmaceutical Company” certification—the top 30% of certified companies by R&D-to-sales ratio will receive additional benefits. MoHW also emphasized support for companies producing essential medicines and exit-prevention medicines.Lee, Kim, and Bae all explained that efforts were also made to include measures to revive the struggling domestic raw material industry within the drug pricing system reform plan. Lee stated, “We hope it will be effective, but it is difficult to predict whether the essential drugs, withdrawal prevention drugs, and domestic raw materials sectors will function properly once the actual reform plan is implemented. We deliberated on creating a drug pricing system that benefits domestic raw material pharmaceutical companies, though some already consider it too late. For now, we will establish a system to protect the domestic industry from a public health security perspective.”Lee specifically outlined a vision to support the smooth prescription and dispensing of unstable-supply drugs on-site, alongside the drug pricing system reform.Specifically, the Ministry of Health and Welfare plans to use prescription-related systems to notify about supply instability and guide substitution with equivalent drugs within the list.Furthermore, the Ministry will establish the legal basis for building and operating a public information system to support post-prescription information sharing between pharmacists and physicians for smooth substitution dispensing, and will also build the information system itself.Lee stated, "When drugs with unstable supply are monitored, we will take administrative action to ensure substitution dispensing occurs actively. Doctors only prescribe the specific drug they intend, and when that drug runs out, they tell the media there's no medicine available. This creates a problem where the public feels anxious even when substitute drugs exist. We have focused significant effort on preventing such situations, ensuring a stable supply of essential medicines, and establishing a supply safety net to avoid triggering public anxiety."
- Policy
- Will incentives for low-price purchasing be expanded?
- by Jung, Heung-Jun Nov 27, 2025 06:14am
- Concerns are emerging that expanding low-price purchase incentives without safeguards could lead to a market collapse, ridden with ultra-low-price bidding wars, eroding the entire pharmaceutical ecosystem. The criticism is that a system focused solely on price competition directly contradicts the government’s stated goals of encouraging pharmaceutical and biotech R&D. According to industry sources on the 25th, the government’s upcoming drug-pricing reform package is expected to include a substantial expansion of low-price purchasing incentives. The low-price purchase incentive is a system where, if a healthcare institution purchases a drug at an actual transaction price lower than the reimbursement ceiling, a portion of the price reduction is provided as an incentive payment. Approximately 20-30% of the price difference is paid to the healthcare institution based on the incentive payment standards announced by the Ministry of Health and Welfare. Although the specific details remain undisclosed, one option being discussed is maintaining the current rule of not lowering drug prices based on actual transaction prices, while increasing the incentive rate to up to 50%. However, there are concerns that even without lowering drug prices based on actual transaction prices, a system that encourages ultra-low-price competition will ultimately lead to a steep decline in profits for manufacturers and distributors. Mr. A from a domestic pharmaceutical company expressed concern, “They are essentially fueling price competition. While I understand the intent to create an ecosystem where competition enables cheaper transactions, it will ultimately lead to a resurgence of 1-won and 2-won bids. Everyone will flock to what is effectively legalized rebates, and the manufacturing ecosystem will collapse.” Critics point out that in a situation where small and medium-sized distributors, including CSOs, proliferate and the lack of transparency in distribution channels is a constant issue, a policy solely focused on encouraging price competition will likely cause more harm than good. The same representative added, “Many injectable drugs have already been through 5 rounds of price cuts based on actual transaction prices, with reductions of around 20% each time. If price competition intensifies further, the damage to injectables will be greater. It’s not enough to simply expand incentives; the government must implement safeguards.” Advice was also given that setting an appropriate minimum bid price would be necessary. Another domestic company representative, Mr. B, stated, “It's hard to predict the outcome, but without setting an appropriate minimum bid price, profits will plummet dramatically. At the very least, it should guarantee cost recovery. Considering the relationship between large hospitals and pharmaceutical companies, they might all rush in with a ‘let's all go down together’ attitude.” Mr. B added, "Furthermore, if incentives rise to 50%, even institutions that have been quiet until now may start making more demands. Currently, payments aren't high enough to warrant claiming the maximum price, but if the reimbursement rate hits 50%, that changes. It may essentially turn into a legalized rebate structure." Some have proposed specific restrictions similar to the minimum-price guarantee applied to Essential Exit-Prevention Drugs, arguing that price-protection mechanisms must accompany any incentive expansion. Mr. A added, “It's necessary to put brakes in place. For injectables with high in-hospital usage rates, we could cap prices at 91% of the drug price, similar to exit prevention drugs. Simply trying to intensify low-price competition is armchair theorizing. Once the ecosystem collapses, it's difficult to recover. The government needs to listen more to real-world concerns.”
- Policy
- Legalizing order for manufacturer to supply essential drugs
- by Lee, Jeong-Hwan Nov 25, 2025 06:13am
- A bill has been proposed in the Korean National Assembly to stipulate the Government Supply System in the Pharmaceutical Affairs Act to ensure the stable supply of National Essential Medicines, which are frequently in short supply due to low profitability. The bill also includes an article expanding treatment opportunities for patients using essential medicines by entrusting the practical work of ensuring a stable supply to the Korea Orphan & Essential Drug Center. On November 24, Democratic Party of Korea Rep. Young-seok Seo announced that he had officially proposed this amendment to the Pharmaceutical Affairs Act. Rep. Seo stated that the number of discontinued products has recently been increasing, primarily in essential medicines with low commercial viability. He noted that the government is currently operating a Government Supply System, such as supporting drug production through agreements with pharmaceutical companies or directly importing overseas pharmaceuticals, when supply is halted due to a company's product withdrawal. However, Rep. Seo expressed concern that the current law does not explicitly provide a legal basis for the Government Supply System, limiting its application and expansion. He further pointed out that, concerning this issue, the legal basis for supporting the resumption of production of medicines not supplied domestically is also inadequate. Therefore, Rep. Seo introduced a bill that legally mandates the Government Supply System for essential medicines and entrusts its practical implementation to the Korea Orphan & Essential Drug Center. In detail, the bill allows the Minister of Food and Drug Safety (MFDS) to mandate a pharmaceutical manufacturer to manufacture National Essential Medicines for products not currently manufactured or imported into Korea when a stable domestic supply is necessary. This bill is to be newly established under Article 41-2, titled 'Manufacture and Supply of National Essential Medicine,' of the Pharmaceutical Affairs Act. In such cases, the MFDS Minister may prepay all or part of the manufacturing costs to the relevant pharmaceutical company. Furthermore, the MFDS Minister can provide administrative and technical support to ensure a swift review process when the company applies for a product license. The MFDS Minister is also mandated to entrust the duties of manufacturing, storage, and supply of National Essential Medicines to the Korea Orphan & Essential Drug Center. The proposed bill also introduces a new article, 'Article 43-2 Emergency Import of Medicines,' into the Pharmaceutical Affairs Act. This new bill allows the MFDS Minister to supply medicines domestically through methods such as importation and provide related information to patients when an urgent domestic supply is deemed necessary or when the head of a relevant central administrative agency or related organizations and groups requests the supply. Rep. Seo explained, "This bill legally specifies the government system for the stable supply of essential medicines and entrusts the practical execution to the Korea Orphan & Essential Drug Center," and added, "It will guarantee treatment opportunities for patients requiring essential medications."
- Policy
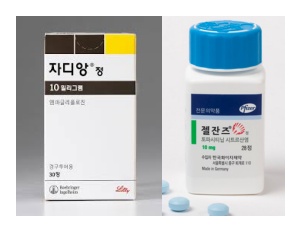
- Price of Jardiance, Xeljanz cut 30% with entry of generics
- by Lee, Tak-Sun Nov 24, 2025 06:19am
- The diabetes treatment Jardiance (empagliflozin, Boehringer Ingelheim) and the rheumatoid arthritis treatment Xeljanz (tofacitinib citrate, Pfizer) will have their maximum insurance prices officially reduced due to the listing of generic versions. When a therapeutically equivalent product is newly listed, the price ceiling is adjusted to 53.55%, and a one-year 70% add-on premium is applied. As a result, starting in December, the previous prices will drop by 30 percentage points. According to industry sources on the 21st, starting December, the price of Xeljanz 5mg Tab will decrease from KRW 10,996 to KRW 7,697, and Xeljanz 10mg Tab will decrease from KRW 18,250 to KRW 12,775. Additionally, the price of Jardiance 10mg Tab will be adjusted from KRW 582 to KRW 408, and Jardiance 25mg Tab from KRW 762 to KRW 533. This price adjustment is due to the inclusion of generic drugs in the reimbursement list. With the substance patent for Xeljanz expiring on the 22nd of this month, 17 products from 12 companies, including those from Daewoong Pharmaceutical and Chong Kun Dang, will be reimbursed starting on the 23rd. Generic versions of Jardiance also entered the market en masse in October. Following the expiration of the substance patent, 235 products from 37 companies were listed for reimbursement on October 24. Both products are achieving high sales in the market. Jardiance recorded KRW 66.3 billion last year based on UBIST data, while the combination product Jardiance Duo recorded KRW 41.8 billion, boasting a combined market size exceeding KRW 100 billion. Xeljanz also secured market presence with KRW 14.3 billion in outpatient prescriptions. The ex officio price reductions made for the extended-release and combination formulations are expected to cause significant concern for Pfizer and Boehringer. Starting December, the price ceiling of Xeljanz XR 11mg will drop from KRW 22,169 to KRW 17,071, and Xeljanz Syrup 1mg/mL will decrease from KRW 527,808 to KRW 369,443. Additionally, the price ceiling of Jardiance Duo Tab 5/1000mg will decrease from KRW 301 won to KRW 284, Jardiance Duo Tab 5/500mg from KRW 301 to KRW 278, and Jardiance Duo Tab 5/850mg from KRW 301 to KRW 278. Among the items subject to this ex officio adjustment, Xeljanz 5mg Tab, Xeljanz 10mg Tab, Xeljanz XR 11mg Tab, and Xeljanz syrup will see an additional price reduction on November 23 next year, once the one-year add-on premium ends, dropping to 53.55%, equal to the generic price. Jardiance 10 mg and 25 mg will undergo the same adjustment on October 24 next year, one year after generic entry. For both original manufacturers, generic competition and reimbursement price cuts will inevitably impact sales, prompting intense efforts to defend market share. Pharmacies are advised to take note of returns and settlement procedures following price changes for these frequently prescribed original products. Meanwhile, drug prices will also be adjusted for the menopausal hormone therapy drug Angelic Tab (drospirenone/estradiol, Bayer) and the antifungal agent Ambisome Inj (liposomal amphotericin B, Gilead) with the entry of their respective generics. The price of Angelic Tab will be adjusted from KRW 10,393 to KRW 5,565 starting in December. The price of Ambisome Inj will be reduced by 30% from KRW 129,392 to KRW 113,218, and after the add-on premium ends on October 1 next year, the price will drop to KRW 86,612.
- Policy
- Scope of use for Kisqali, Imfinzi expanded for combo use
- by Jung, Heung-Jun Nov 21, 2025 06:12am
- Use of combination therapies for breast cancer, endometrial cancer, urothelial carcinoma, and prostate cancer has been newly approved, expanding the expected treatment scope for drugs such as Kisqali and Imfinzi. Starting December, 5 new combination therapies for breast cancer, 2 for endometrial cancer, and 1 each for urothelial and prostate cancer will be established. The Health Insurance Review and Assessment Service (HIRA) is collecting industry opinion on the revision of the notification regarding drugs prescribed and administered to cancer patients until the 24th. For breast cancer, four new combination therapies using Novartis Korea's breast cancer treatment Kisqali (ribociclib) tablets will be added. Newly established methods include ribociclib + anastrozole, ribociclib + letrozole, and adding leuprorelin (LHRH agonist). Additionally, the non-reimbursed triple combination of inavolisib + palbociclib + fulvestrant—will also be added.. This option is used when cancer progresses despite treatment with other targeted therapies. Combination therapies using AstraZeneca's immunotherapy Imfinzi (durvalumab) for endometrial cancer and urothelial carcinoma will also be expanded. For endometrial cancer, two new regimens are introduced: either durvalumab monotherapy following combination therapy with durvalumab + carboplatin + paclitaxel, or durvalumab + Olaparib combination therapy. For urothelial carcinoma, the approach involves combination therapy with durvalumab + gemcitabine + cisplatin followed by radical cystectomy, with durvalumab used as adjuvant therapy. For both endometrial cancer and urothelial carcinoma, the addition of durvalumab to existing reimbursed regimens (carboplatin + paclitaxel, gemcitabine + cisplatin) will be added. For prostate cancer, a new combination therapy combining the non-reimbursed talazoparib with the reimbursed enzalutamide has been newly established. The newly established combination therapies expand treatment options using targeted therapies and immunotherapies based on genetic mutations and DNA repair capabilities. HIRA also revised reimbursement criteria for 19 items among Groups 1-2 anticancer drugs. This aims to clarify reimbursement standards for therapies that are not considered standard treatments. Changes, such as adding or deleting phrases, were made to the reimbursement criteria for anticancer drugs used in malignant melanoma, rhabdomyosarcoma, neuroblastoma, Wilms tumor, and retinoblastoma. HIRA stated, “We have announced additional combination therapies requested by academic societies that were deemed to comply with the general principles of the official notice and announcement.”
- Policy
- Ineffective dual pricing system to be revised
- by Jung, Heung-Jun Nov 20, 2025 06:16am
- The government has acknowledged the failure of the 'Separate Contracting System: Dual Pricing System', implemented last March to support the export of domestically developed new drugs. It will soon introduce a revised plan under a new name. The revised plan is expected to be submitted as an agenda item to the Health Insurance Policy Review Committee meeting on November 28 of this month, with the option to allow dual pricing for drugs other than those for severe and rare diseases being strongly considered. According to industry sources November 19, the government plans to significantly relax requirements for the dual pricing system, including conditions for ▲new drugs developed by companies that received Innovative Pharmaceutical Companies designation ▲eligibility for expedited review ▲domestic clinical trial requirements. Currently, no product meets all three requirements to use the dual pricing system. The system, though well-intentioned to support the development of domestic new drugs, has essentially remained a regulation without practical use. An official from pharmaceutical company A explained, "There were many restrictions. The number of new drug developments by domestic companies is at a low point, and few multinational pharmaceutical companies have received innovativeness designation," and added, "Moreover, the target for expedited review is often narrowed to severe and rare disease drugs, meaning ultimately, there are no drugs utilizing the dual pricing system." Currently, 45 domestic pharmaceutical and biotechnology companies of South Korea and four multinational pharmaceutical companies have received the innovativeness designation. Only drugs held by these companies that are also eligible for expedited review and have undergone domestic clinical trials could apply for the dual pricing system. Multinational pharmaceutical companies also assessed the original dual pricing system as insufficiently incentivizing. Since many multinational companies already utilize the refund-type Risk-Sharing Agreement (RSA), they were not interested in the dual pricing system, which offered no additional benefit. An official from multinational pharmaceutical company B assessed, "When the policy direction came out, we thought it would be widely used, but when we actually looked into it, there was no major advantage," and added, "There was many opportunity to utilize the RSA refund type, and since (applying the dual pricing system) did not grant an exemption from health-economic evaluation, it was difficult to see any added value." Following criticism in the National Assembly that the system lacked practical effectiveness due to zero uptake, the government is revising the regulation and renaming it the 'Flexible Drug Pricing Contracting System'. To avoid repeating the mistake of merely changing the name without addressing the lack of efficacy, incorporating field feedback is critical. The National Health Insurance Service (NHIS) is currently conducting internal reviews and gathering industry opinions for the introduction of the Flexible Drug Pricing Contracting System. Prospective expansion for eligible drugs for the dual pricing...will be gradually introduced to RSA drugs Even before the government announced the finalized plan, there was already discussion about the prospective expansion of the drugs eligible for the dual pricing system. Some predict that market-entry hurdles, such as the requirement for innovativeness designation and eligibility for expedited review, will be entirely removed. A multinational company official said, "The direction will likely be to allow it even for companies outside of the innovativeness designation. We anticipate that a wide range of products from pharmaceutical companies that show commitment will be permitted." The official also predicted, "We expect that RSA drugs will switch to the dual pricing system when their cost-sharing period ends or when the listing of a subsequent drug mandates the disclosure of their official listed price." Domestic pharmaceutical companies preparing for overseas export are also awaiting the government's detailed plan. They are, however, optimistic about the decision to expand eligibility. A domestic company official stated, "Since there are countries that reference our drug prices, it is beneficial if the official external price is higher. If implemented, we plan to enter the flexible contract system," and added, "Although the number of items for domestic companies is limited, it is much better than nothing. We will have to wait and see exactly how much they relax the rules, but I understand they are going to expand the scope of application proactively." This official estimated, "They seem to be aiming for implementation around the second half of next year, after consulting with the industry for necessary adjustments." Another industry official commented, "Given the external issues, the Lee Jae Myung administration is also interested in the dual pricing system, so I think it will be discussed as a priority item in the drug pricing reform." The commenter strongly suggested that the plan be submitted to the committee on the 28th of this month. The Ministry of Health and Welfare (MOHW) is being cautious ahead of the official announcement. A MOHW official said, "We are preparing improvements, and because the announcement will be made soon, it is difficult to disclose the details," and added, "We are discussing with the personnel in charge at the executive agency (NHIS) and are in the process of gathering opinions from stakeholders regarding the system improvement."
- Policy
- Temporary relaxation of reimb criteria for Dupixent ends
- by Jung, Heung-Jun Nov 20, 2025 06:15am
- The temporary relaxation of reimbursement requirements for certain drugs, which was implemented since April last year due to the medical staffing crisis, will end this year. Drugs like Dupixent, MabThera, and Soliris must resume the patient response assessments, which the drugs were granted exemption from, starting January next year. According to industry sources on the 19th, the Ministry of Health and Welfare (MOHW) will end the temporary relaxation of drug reimbursement requirements as a follow-up measure after lifting the severe-level health crisis alert. When a shortage of medical staff occurred due to the resident physician walkout, medications requiring pre-administration tests and evaluations were omitted at the discretion of treating physicians. Frontline clinicians had requested relief from strict reevaluation requirements that mandated reassessment before renewing prescriptions. In recognition of the practical difficulties in adequately conducting patient tests and evaluations, the reimbursement criteria were temporarily relaxed as an unavoidable measure. The alert was lifted on October 20th as the medical staffing shortage was resolved. However, to prevent confusion in clinical settings, a grace period of approximately 2 months was granted before ending the reimbursement relaxation. A wide range of medications are affected, including anticancer drugs, epilepsy treatments, dementia drugs, stimulants, and atopic dermatitis therapies. High-profile injectables such as Dupixent, MabThera, Soliris, Eylea, Humira, and Spinraza will once again require documented patient evaluations. Oral agents such as Xeljanz for autoimmune disease, Concerta OROS for ADHD, and Opsumit for pulmonary arterial hypertension are also included. These medications are covered under insurance only if administered based on test results. Periodic evaluations, such as every 3 to 6 months, must be conducted to qualify for coverage. For example, initial and maintenance treatment criteria often require submission of objective evidence such as prior treatment history, EASI score calculation, and lesion photographs. Beginning January, reimbursement will once again be contingent upon meeting such evaluation requirements, and providers are advised to exercise particular caution.