- LOGIN
- MemberShip
- 2025-12-19 08:22:11
- Policy
- Korea’s 2nd GIFT drug Nefecon is approved in KOR
- by Lee, Hye-Kyung Nov 22, 2024 05:55am
- Meditip's Nefecon (budesonide), which was designated as the 2nd Global Innovative Products on Fast Track (GIFT) drug, has been approved in Korea. The Ministry of Food and Drug Safety approved Nefecon on the 18th. Nefecon is used to treat primary IgA nephropathy in adults with a urinary protein-to-creatinine ratio of 1.5 or greater who are at risk of rapid progression. Budesonide, the main ingredient, is already on the market and in use in Korea, but the indication Meditip applied for budesonide's approval as a treatment for new patients who have no existing treatment option, which is why the government designated it as a GIFT drug in March last year. Nefecon was approved by the U.S. FDA on December 15, 2021, as an accelerated review (AA) and priority review (PR) drug, and was approved by the European Medicines Agency (EMA) on July 15, 2023, as an accelerated assessment (AA) and conditional marketing authorization (CMA) drug. In China, the National Medical Products Administration (NMPA) designated Nefecon as a Breakthrough Therapy Designation (BTD) in 2020, and the Taiwan Food and Drug Administration granted it an Accelerated Drug Designation (ADD). In Korea, it was designated as an orphan drug on November 17, 2022. In a presentation made at Kidney International, Nefecon was associated with a 27% lower urinary protein-to-creatinine ratio at 9 months of treatment compared to placebo. The glomerular filtration rate remained stable, with a difference of 3.87 ml/min/1.73㎡ compared with placebo. IgA nephropathy is a disease caused by the deposition of immune complexes, including IgA, in the glomeruli of the kidneys, causing an inflammatory response. About 9,000 patients are known to be affected by IgA nephropathy in Korea. In clinical practice, antihypertensive drugs such as ARBs and ACEIs, immunosuppressants, and diuretics are used to treat IgA nephropathy. However, these drugs are symptomatic treatments that prevent the worsening of symptoms, and there is no drug that fundamentally treats the condition.
- Policy
- CKD drug Kerendia seeks indication expansion in KOR
- by Lee, Hye-Kyung Nov 21, 2024 05:46am
- Bayer Korea's Kerendia (finerenone), which is used to treat adult patients with chronic kidney disease with type 2 diabetes, will enter Phase III clinical trials in Korea to expand its indication. On the 20th, the Ministry of Food and Drug Safety (MFDS), approved a ‘randomized clinical trial to determine the efficacy and safety of finerenone on the morbidity and mortality of heart failure patients with left ventricular ejection fraction greater than 40% who were hospitalized for acute non-targeted heart failure episodes.’ Kerendia, which received domestic approval in May 2022, is indicated for the sustained reduction in estimated glomerular filtration rate (eGFR), progression to end-stage renal disease, and reduction in the risk of cardiovascular death, nonfatal myocardial infarction, and hospitalization for heart failure in adult patients with chronic kidney disease with type 2 diabetes. Kerendia is a first-in-class, orally administered, selective, nonsteroidal mineralocorticoid receptor antagonist (MRA) that has a novel mechanism that inhibits kidney inflammation and fibrosis in adult chronic kidney disease patients with type 2 diabetes. In addition, Kerendia has recently been shown to prevent heart failure-related secondary events in HF with mildly reduced ejection fraction (HFmrEF) and HF with preserved ejection fraction (HFpEF) with a left ventricular ejection fraction (LV ejection fraction) of 40% or greater and has entered into global Phase III trials. The clinical trial to expand the indication to heart failure has also been approved in Korea. Meanwhile, results from the Phase III FINEARTS-HF trial, which evaluated Kerendia in heart failure patients with left ventricular ejection fraction greater than 40%, were presented at the European Society of Cardiology Annual Congress 2024 (ESC 2024) in September. Data from the Phase III FINEARTS-HF study showed that at a median follow-up of 32 months, there were a total of 1,083 worsening heart failure events in 624 of the 3003 patients in the Kerendia arm and a total of 1283 events in 719 of 2998 patients in the placebo arm. The total number of worsening heart failure events was 842 in the Kerendia arm and 1024 in the placebo arm, with an 18% lower incidence rate in the Kerendia arm. In addition, the proportion of patients who died from cardiovascular causes was 8.1% and 8.7%, respectively, with a 7% lower hazard ratio observed in the Kerendia arm. However, there was no significant difference between death from cardiovascular events and all-cause mortality. According to drug research institution UBIST, prescriptions for Kerendia totaled KRW 1 billion in the first half of this year, and Bayer Korea is expected to expand the indications to further gain a competitive advantage.
- Policy
- Strengthed regulations on human drug use in vet clinics
- by Lee, Jeong-Hwan Nov 20, 2024 06:08am
- A bill that requires frontline pharmacies to record detailed distribution details on human specialty drugs sold to veterinary hospitals has passed the Bill Review Subcommittee review. Although there was a lot of disagreement over the bill between the professions subject to the regulation - the pharmacists and the veterinarians - the Health and Welfare Committee’s Bill Review Subcommittee members agreed on the need to address the issue of misuse of human specialty drugs in veterinary hospitals. On the 19th, the 1st Bill Review Subcommittee of the National Assembly's Health and Welfare Committee reviewed and voted, and passed the bill to amend the Pharmaceutical Affairs Act, introduced by Representative Young-Seok Seo of the Democratic Party of Korea. The core of Seo's bill is to impose reporting obligations on pharmacists when they sell specialty drugs for human use to veterinarians at veterinary hospitals. The bill establishes a distribution management system that requires pharmacists to report their sales to the Korea Pharmaceutical Information Center every time they sell specialty drugs to veterinarians to prevent misuse and abuse of human drugs through veterinary hospitals. In addition, when pharmacists sell specialized drugs to veterinarians, they are required to submit the name of the veterinary hospital, contact information, drug name, quantity, and date of sale to the Korea Pharmaceutical Information Center in accordance with the Ministry of Welfare decree. If a pharmacist fails to submit the sales details or submits them falsely, he or she will be fined up to KRW 1 million. In particular, the bill mandates the computer network of the Korea Pharmaceutical Information Center to be linked to the veterinary prescription management system operated under the Veterinarians Act to transparently manage the distribution channels of specialty drugs sold to veterinarians. The Korean Pharmaceutical Association submitted a position in favor of the bill and the Korean Veterinary Medical Association submitted a position against the bill, but the Bill Review Subcommittee decided to pass the bill. Although the bill passed the Bill Review Subcommittee, it remains to be seen whether it can pass the Legislation and Judiciary Committee as the two professions are at odds. Earlier, Rep. Seo explained the background of the bill, saying that the process of selling specialized drugs for human use to veterinary clinics is causing issues of misuse and abuse, while at the same time, 'drug delivery,' which is prohibited by the current law, is also being carried out covertly.
- Policy
- Stelara biosimilars face increasing competition
- by Lee, Tak-Sun Nov 20, 2024 06:08am
- Product photo of Janssen Competition among biosimilars referencing Stelara (ustekinumab) for treating autoimmune diseases heats up. Samsung Bioepis added its biosimilar to the reimbursement list in July, and Celltrion added theirs in September. Since Celltrion is set to cut the price starting next month, attention has been drawn to future competition. According to industry sources on November 19, Celltrion will cut the prices of existing products after their Steqeyma IV Inj, an intravenous formulation (IV), becomes listed for reimbursement in December. In September, Celltrion listed two Steqeyma PFS products for reimbursement, although the IV formulation was not included then. In contrast, the original Janssen Stelara and Samsung Bioepis Epyztek are available on the market with a total of three formulations, including the IV formulation. With the launch of Celltrion's IV formulation, the company has positioned well agaisnt the competitors. Additionally, by cutting its drug price, Celltrion has gained a competitive edge in pricing. The ceiling price for Steqeyma IV is KRW 1,278,313, making it more affordable than comparable formulations from Janssen and Samsung Bioepis. Janssen's Stelara S.C. Inj is priced at KRW 1,809,200, and Samsung Bioepis's Epyztek IV Inj is set at KRW 1,345,593. This makes Steqeyma approximately KRW 530,000 cheaper than the original product and about KRW 70,000 less expensive than the Samsung Bioepis formulation. Additionally, Celltrion has voluntarily reduced the price of its prefilled syringe formulation. Steqeyma PFS 45 mg price has been lowered from KRW 1,298,290 to KRW 1,233,376. For comparison, Janssen’s Stelara S.C. Inj·Stelara PFS are priced at KRW 1,745,600, while Samsung Bioepis’s Epyztek PFS is priced at KRW 1,298,290. This makes Celltrion’s product approximately KRW 510,000 cheaper than the original and about KRW 60,000 less expensive than Samsung Bioepis’ product. Additionally, the Steqeyma PFS 90 mg price has been reduced from KRW 1,342,320 to KRW 1,275,204. Currently, the original product is priced at KRW 1,804,800, and Samsung Bioepis' product is priced at KRW 1,342,320. Celltrion's prefilled syringe products were previously priced the same as Samsung Bioepis' products. However, Celltrion has officially entered the price competition with the recent price reduction. The price of the original Stelara will decrease to the KRW 1,500,000 range after its additional reimbursement ends in July next year. However, there will still be a significant gap compared to Celltrion's product, giving Celltrion an advantage in price competitiveness. According to IQVIA, Stelara's sales reached KRW 47.4 billion last year. It is reimbursed for treating moderate-to-severe plaque psoriasis in adults and children aged 12 and older, active psoriatic arthritis in adults, active Crohn's disease in adults, and moderate-to-severe ulcerative colitis in adults.
- Policy
- Samil voluntarily cuts price of its Vemlino to lowest price
- by Lee, Tak-Sun Nov 19, 2024 06:13am
- Samil Pharm is voluntarily reducing the price of its hepatitis B drug Vemlino (Tenofovir Alafenamide Hemimalate). This will render the drug to be the lowest-priced drug among tenofovir alafenamide drugs, increasing its price competitiveness. According to industry sources on the 18th, the price of Vemlino will be reduced from KRW 2,425 to KRW 2,358 starting next month. There are currently 9 tenofovir alafenamide-based hepatitis B treatments, including the original Vemlidy (tenofovir alafenamide hemifumarate, Gilead). The other 8 are salt-modified agents. Vemlidy is an upgraded version of Gilead's Viread. Viread was associated with side effects of decreased kidney function or bone density in some patients, so the upgraded Vemlidy has become the most commonly recommended initial treatment on site as it contains one-tenth the active ingredient dose of Viread while providing the same level of viral suppression. The salt-modified versions of Vemlidy have been sequentially released to the market starting with Dong-A ST’s ‘Vemlia Tab (tenofovir alafenamide citrate). The companies were able to enter the market by avoiding patents through salt modification. However, even with the introduction of such versions, the original's status remained unchanged. Vemlidy’s sales continued to soar, reaching KRW 27.9 billion in 2021, KRW 39.3 billion in 2022, and KRW 49.2 billion in 2023, according to UBIST. This is the third consecutive year that the drug’s upper limit has been reduced through the price-volume agreement negotiations due to increased usage. The price of Vemlino, whose price was lowered this time, was only KRW 300 million (UBIST) last year. Samil had listed its drug at KTW 2,425, lower than the calculated amount when it was listed in July last year, to gain price competitiveness. However, the lowest price title went to Dongkook Pharmaceutical’s Alfoterin Tab (Tenofovir Alafenamide Hemimalate). The upper limit for Alfoterin is KRW 2,424. This time, Samil lowered the price of Vemlino to KRW 2,328 and took over the lowest price title. The highest price for tenofovir alafenamide is currently KRW 3,235 for the original Vemlidy Tab, and the difference between Vemlidy and Vemlino is KRW 887. HBV patients are sensitive to the price because they need to take medications for life. Therefore, it will be interesting to see if Vemlino can gain market share with its competitive price. An industry insider said, “The market for tenofovir alafenamide preparations has only 8 generic contestants that entered the market through patent evasions, so there is less competition and the companies can take more of the market share. However, the generic companies are struggling due to high dependence on the original, and the eyes are on whether the low-price competition can break this pattern.”
- Policy
- HK Inno.N to distribute Tamiflu as Roche’s new partner
- by Lee, Tak-Sun Nov 18, 2024 05:49am
- HK Inno.N will be responsible for the domestic distribution of ‘Tamiflu Cap’ in addition to the distribution of the antiviral drug ‘Xofluza.’ The company is expected to gain external growth with the distribution of Tamiflu, which has an annual sales volume of KRW 15 billion. According to industry sources on the 15th, HK Inno.N sent a letter to its business partners and announced that it will distribute Roche Korea’s Tamiflu Cap. 30/45/75mg from the 14th. Tamiflu is “the” antiviral flu drug. Its sales were sluggish in 2020 and 2021 during the height of the COVID-19 pandemic, but have risen significantly since last year with the rise of the flu epidemic. Last year, its outpatient prescriptions amounted to KRW 15 billion based on UBIST. Its previous distributor, Chong Kun Dang had distributed Tamiflu in Korea since 2012, but the companies’ partnership ended last year and Roche has been seeking a new domestic partner since. Last year, HK Inno.N signed an exclusive domestic distribution and commercialization agreement with Roche Korea for the antiviral flu drug Xofluza (baloxavir marboxil). Under the agreement, HK Inno.N will distribute Xofluza for 2 years and jointly conduct marketing and sales activities with Roche Korea. Xofluza is a new drug that can treat influenza with just one dose, unlike other oral influenza treatments that require 5 days of administration. It is the first flu antiviral drug to be developed in 20 years. However, Xofluza is still non-reimbursed in Korea, so it has not been able to raise significant domestic sales. If it succeeds in being reimbursed in Korea, it is expected to take over Tamiflu's sales. Roche had shown its trust in HK Inno.N, signing for Tamiflu following Xofluza. HK Inno.N is expected to become Roche's new partner in Korea after Chong Kun Dang. “HK Inno.N has recently emerged as a key player in the domestic respiratory virus drug market, supplying Pfizer's new COVID-19 vaccine,” said a pharmaceutical industry insider. ”Its distribution of Tamiflu will help accelerate the company's external growth and raise company awareness in the antiviral drug market.”
- Policy
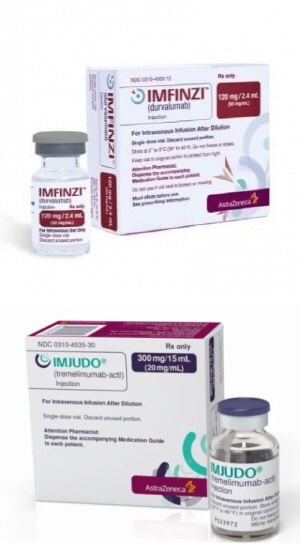
- AZ’s Imjudo and Imfinzi both make reimbursement progress
- by Lee, Tak-Sun Nov 15, 2024 05:49am
- AstraZeneca (AZ) Korea’s ‘Imjudo Inj.’ Has passed the first step to reimbursement listing in Korea. Also, reimbursement standards for ‘Imfinzi Inj,’ which is seeking reimbursement expansions, were set. However, these are only the first steps to reimbursement. On the 13th, the Health Insurance Review and Assessment Service held the 8th Cancer Disease Review Committee in 2024 to deliberate on the reimbursement standards for anticancer drugs. As a result of the deliberation, reimbursement standards were set for the new drug Imjudo Inj (tremelimumab) but not for Tecvayli Inj (teclistamab, Janssen). Imjudo is indicated for the first-line treatment of adult patients with advanced or unresectable HCC in combination with durvalumab. Here, durvalumab is AZ's immuno-oncology drug Imfinzi. Imfinzi is indicated for the treatment of patients with unresectable locally advanced non-small cell lung cancer (NSCLC) whose disease has not progressed after platinum-based concurrent chemoradiotherapy (CCRT). However, based on clinical results, the company has been seeking to expand its coverage to include liver and biliary tract cancers. One such example is the Imfinzi-Imjudo combination as a first-line treatment for liver cancer. Another is biliary tract cancer. Since February, Imfinzi+gemcitabine+cisplatin has been used for the first-line treatment of patients with locally advanced or metastatic biliary tract cancer. However, Imfinzi is currently paid fully out-of-pocket. As Imfinzi is an expensive drug, costing KRW 3,347,202 per bottle, healthcare professionals as well as patients have been requesting Imfinzi be covered by the government. The patients' voices were also discussed as an agenda at the last national audit. In response, HIRA had promised a proper review. On this day, their efforts bore fruit. Reimbursement standards were finally set for the drugs. With both drugs being set reimbursement standards, AZ can now look forward to the prompt reimbursement of both of its drugs. However, another AZ drug - Tagrisso (osimertinib mesylate), the company's flagship treatment for non-small cell lung cancer- failed to make the cut. The company sought to set reimbursement standards for Tagrisso in combination with pemetrexed plus platinum-based chemotherapy in the first-line treatment of patients with locally advanced or metastatic non-squamous NSCLC with an EGFR exon 19 deletion or exon 21 (L858R) substitution mutation, but the application was not accepted. On the same day, the CDDC deliberated on reimbursement standard improvements proposed by medical societies. As a result, requests to improve the phrase - “failed chemotherapy including docetaxel” - wording in the subjects for prostate cancer chemotherapy, and suggestions regarding the administration of palliative therapy in case of recurrence/metastasis during or after the administration of adjuvant therapy, were accepted.
- Policy
- NHIS-Roche enters pricing negotiations for Perjeta’s reimb
- by Lee, Tak-Sun Nov 12, 2024 05:50am
- Roche has started drug price negotiations with the National Health Insurance Service for its breast cancer drug Perjeta (pertuzumab). The negotiations are expected to be held on the drugs whose reimbursement standards were set by the Cancer Disease Review Committee in May. According to industry sources on the 11th, the NHIS recently included Perjeta in its list of drug price negotiation subjects in November. The items subject to drug price negotiation are new drugs, drugs that are not subject to drug price negotiations, and drugs with expanded use. This negotiation appears to be a case of expanded scope of use, as Perjeta was already listed for reimbursement in June 2017. In its May meeting, the NHIS’s Cancer Disease Review Committee set reimbursement standards for Perjeta. Currently, Perjeta is reimbursed for HER2-positive metastatic or unresectable locally recurrent breast cancer. It also applied a 30% coinsurance rate as an adjuvant therapy for breast cancer. The newly reimbursed indication is for lymph node-positive HER2-positive patients who are eligible for pertuzumab-based neoadjuvant therapy. The reimbursement standard was established according to recommendations from the medical community. In December last year, HIRA received feedback from medical associations, including the Korean Medical Association and the Korean Hospital Association, regarding its reimbursement criteria. The proposed item for anti-cancer drugs was then discussed in detail by the TFT and was presented to the Cancer Disease Review Committee in June. In breast cancer, pertuzumab is the only drug for the indication. Clinical trials have shown the effectiveness of pertuzumab in patients with lymph node-positive breast cancer. However, in the past, only neoadjuvant therapy with pertuzumab for early breast cancer patients was covered with selective reimbursement, and adjuvant therapy was not covered, so many voices were calling for reimbursement expansions. In that context, the newly set reimbursement standards are an example of access being strengthened through the voice of the field. However, the final expansion of reimbursement benefits will be finalized only after completing negotiations with the National Health Insurance Service. “Recently, the reimbursement expansions proposed by the medical community and academic societies, not pharmaceutical companies, are entering the negotiation stage,” said an NHIS official, explaining, ”We are closely examining the agendas because the measures for sharing those drugs’ financial burden has not been sufficiently discussed yet.” Meanwhile, Perjeta continues to build on its reputation as a blockbuster breast cancer drug that generated KRW 111.3 billion in sales last year, according to IQVIA. Roche recently launched Phesgo, a combination of Perjeta and Herceptin, to continue to expand its share of the breast cancer treatment market.
- Policy
- Drug switching for atopic dermatitis measure submitted
- by Lee, Tak-Sun Nov 07, 2024 05:47am
- Pharmaceutical companies have submitted their measures for voluntary drug price reduction as part of financial allotment following expanded reimbursement. Discussions about allowing drug switching between biological agents and JAK inhibitors used to treat severe atopic dermatitis are speeding up. The Health Insurance Review and Assessment Service (HIRA) has already prepared the reimbursement criteria, and pharmaceutical companies have submitted their measures for voluntary drug price reduction as part of financial allotment following expanded reimbursement. Attention is drawn to whether it will pass the Drug Reimbursement Evaluation Committee (DREC) review, the final stage for reviewing reimbursement appropriateness. According to industry sources on November 6th, pharmaceutical companies Pfizer for Cibinqo and Lily for Olumiant submitted their financial impact analysis to the HIRA. The drug pricing reduction system for expanded scope of use requires the submission of financial impact analysis reports. The government excludes drug price reduction when the estimated additional claim amount following the expanded scope of use is below KRW 1.5 billion. The drug price reduction is applied for products over KRW 1.5 billion and below KRW 10 billion. In this case, pharmaceutical companies are required to submit a financial impact analysis, including measures to voluntarily lower prices, to the HIRA. In mid-October, the HIRA required each pharmaceutical company to submit financial impact analysis. Earlier this month, pharmaceutical companies have started to submit their reports. The issue of allowing drug switching for the treatment of severe atopic dermatitis was dealt with in the parliamentary audit held last month, so the health and welfare authority appears to be rushing the measure. The HIRA has been discussing with experts since September about whether to allow drug switching between biological agents and JAK inhibitors. The reimbursement criteria have been prepared, reflecting the latest evidence and clinical application. The remaining step is to minimize the financial impact following the expanded scope of use. Pharmaceutical companies' percentage of voluntary drug price reduction is the key. As pharmaceutical companies have submitted their measures for voluntary price reduction, the the Drug Reimbursement Evaluation Committee (DREC) review of the HIRA will make a final decision. The 11th DREC review is scheduled for July 2024. Attention is drawn to whether drug switching for severe atopic dermatitis will be considered for the agenda. When it passes the DREC review, the final reimbursement expansion will be decided through negotiation with the National Health Insurance Service (NHIS). Meanwhile, treatments for severe atopic dermatitis have been listed for reimbursement, starting with the biological agent, 'Dupixent Inj,' approved in January 2020. It was followed by the JAK inhibitors, Olumniant Tab, Linvoq ER Tab, and Cibinqo Tab. In May, another biological agent, Adtralza PFS, was listed for reimbursement. As a result, patients have more drug options. However, reimbursement and special cases do not cover drug switching between biological agents and JAK inhibitors, so it remains an issue. When clinical practices initially use high-cost drugs, and then the effects seen are not apparent, they may continue administering first-line treatments. Therefore, the medical community has been demanding the allowance of drug switching.
- Policy
- Novartis cuts price of Cosentyx after PVA negotiations
- by Lee, Tak-Sun Nov 07, 2024 05:46am
- Cosentyx UnoReady Pen The price of Novartis’s Cosentyx(secukinumab) is set to be reduced due to its increased use. This is because the National Health Insurance Service recently reached an agreement during Price-Volume Agreement negotiations with the company for Cosentyx. According to industry sources on the 6th, the insurance price ceiling of Cosentyx was adjusted for the second time under the PVA system after 2021. The affected products are the Cosentyx SensoReady Pen and Cosentyx UnoReady Pen. Cosentyx was listed for reimbursement in Korea in August 2017. Currently reimbursed indications include ▲chronic severe plaque psoriasis, ▲active and progressive psoriatic arthritis, and ▲severe active ankylosing spondylitis. According to the market research institution IQVIA, Cosentyx SensorReady Pen has seen an increase in sales every year since its reimbursement. In 2019, sales reached KRW 12.3 billion, then KRW 19.9 billion in 2020, KRW 25.1 billion in 2021, KRW 30.3 billion in 2022, and KRW 31.7 billion in 2023. In addition, Cosentyx UnoReady Pen, a high-dose product that was launched last year, generated sales of KRW 4.5 billion. Due to such high demand, the insurance price ceiling of Cosentyx had been adjusted in 2021 under the PVA system. At the time, the price of Cosentyx Sensoready Pen dropped by 7.3% from KRW 682,938 to KRW 630,084. At the time, 'Type A’ of the PVA negotiations was applied. Type A negotiations are applied to listed new drugs whose claims in the same product category have increased by more than 30% from the expected claims amount. In 2020, the drug had already well exceeded the expected claims amount. Three years have passed, and this year, ‘Type B’ PVA negotiations were applied. Type B negotiations are applied when the claims for the same product group, whose insurance price ceiling had been adjusted by Type A negotiations, or four years passed after the date of initial listing or the date the price ceiling was adjusted through negotiations, have increased by 60% or more than the previous year's claims, or by 10% or more but the increase is more than KRW 5 billion. Since Cosentyx had its price ceiling adjusted through Type A negotiations, it is possible that the additional negotiation for price adjustments was ordered because its claims increased by more than 60% or more than KRW 5 billion over the previous year's claims. Given that Cosentyx Sensoready’s sales grew 5% YoY in 2023 by KRW 1.4 billion (IQVIA), the introduction of Cosentyx UnoReady Pen, which falls in the same product group, may have triggered the PVA negotiation. Combined, the increase in sales amounted to more than KRW 5 billion compared to the previous year. Upon the agreement, the final price of Cosentyx is expected to be announced after deliberation by the Ministry of Health and Welfare’s Health Insurance Policy Review Committee.