- LOGIN
- MemberShip
- 2025-12-17 15:49:08
- Policy
- 'Photos' to be excluded from files for negotiated drugs
- by Jung, Heung-Jun Nov 19, 2025 06:08am
- "Some companies submitted product photos and test certificates, but their production records were not found the following year. This must not happen." The National Health Insurance Service (NHIS) made this statement during a recent drug price consultation session with pharmaceutical industry stakeholders, announcing future institutional changes. According to industry sources on November 18, the NHIS is gathering opinions on changes to the documentation required for negotiated drugs and the scope of disclosure for Risk-Sharing Agreement (RSA) drugs, until November 21. The main point of the changes to the negotiated drug documentation is to replace product photos with the Manufacturing Instruction Record. The goal is to prevent instances such as the submission of a semi-finished product test certificate or the forgery of a finished product test certificate when no finished product inventory is actually available for shipment. Consequently, product photos will not be required unless requested. The primary objective is to substitute these with the Manufacturing Instruction Record to secure credibility. The NHIS plans to conduct an internal review in December and apply these changes starting with the main negotiations for negotiated drugs in January next year. The NHIS is also pursuing the disclosure of information regarding RSA drugs. The NHIS is also seeking opinions on the scope of information to be disclosed, including the drug name and type, within the same deadline. Some in the National Assembly have argued that related information must be disclosed to ensure the transparent operation of the RSA system. The NHIS is seeking opinions on two options: Option 1, which discloses only the drug name, and Option 2, which discloses both the drug name and the RSA type. The NHIS is also gathering general opinions on the overall disclosure of RSA drug information. The NHIS plans to disclose drug name and the contractually set refund rate to subsequent reimbursement applicants. An NHIS official previously stated, "We believe it is right to disclose the refund rate information for RSA drugs. We will ensure that this information is provided upon request when a reimbursement application is submitted to HIRA. However, the requesting applicant must sign a non-disclosure agreement."
- Policy
- World’s first dutasteride+tadalafil combo to launch in Dec
- by Lee, Tak-Sun Nov 19, 2025 06:07am
- The world’s first dutasteride and tadalafil combination approved for benign prostatic hyperplasia (BPH) is expected to be released next month as a non-reimbursed product. This effectively confirms that the companies failed to secure reimbursement for their respective products. According to industry sources on the 17th, the dutasteride+tadalafil fixed-dose combination will begin sales next month at an expected price of KRW 1,300. Four products from four companies received approval: Dong-A ST's Dutana Tab 0.5/5mg, Shinpoong Pharm’s Avocial Tab 0.5/5mg, Dongkook Pharmaceutical's Uresco Tab 0.5/5mg, and DongKoo Bio&Pharma's Uroguard Tab 0.5/5mg. The four drugs were approved on January 23rd through joint development. The approved indication is the treatment of moderate to severe symptoms of benign prostatic hyperplasia. Dongkook Pharmaceutical is the manufacturer. These companies received approval in January and have been pursuing reimbursement listing. However, because tadalafil is a non-reimbursed ingredient, the combination could not be priced under standard calculations and would have had to enter the new drug reimbursement track, which requires substantial data submission and a prolonged evaluation period. Ultimately, manufacturers agreed to proceed with a non-reimbursed market launch. Some companies are informing distributors and retailers of their new product launch plans for December. The KRW 1,300 price is viewed as reasonable. Currently, the maximum price for dutasteride tablets 0.5mg is KRW 709. Given that the lowest price for tadalafil 5mg is around KRW 700, the combination drug’s price is lower than the sum of the two components. Still, since most BPH treatments in Korea are reimbursed, it remains uncertain how much demand there will be for a non-reimbursed option. Industry observers note that manufacturers may lean toward highlighting tadalafil’s erectile dysfunction benefits as a marketing angle.
- Policy
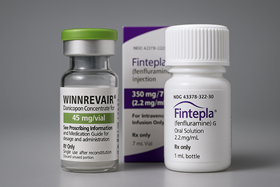
- First batch of drugs gain reimb through AEN pilot project
- by Jung, Heung-Jun Nov 17, 2025 06:11am
- With the first drugs under the Approval-Evaluation-Negotiation pilot program all securing reimbursement, the timing for the second batch of drugs currently under review to enter reimbursment listing is also approaching. Expectations are building for the possibility of their sequential insurance coverage as early as the first half of next year. With Bylvay Cap (odevixibat) gaining reimbursement coverage last month, all first-phase drugs under the Approval-Evaluation-Negotiation (AEN) project have now entered Korea’s reimbursement system, joining the previously covered Qarziba Inj (dinutuximab beta). The second batch of products under review are Winrevair (sotatercept) by MSD Korea for pulmonary arterial hypertension, Fintepla (fenfluramine) by UCB Korea for Dravet syndrome, and Limcato (anbal-cel) by the domestic company Curocell for large B-cell lymphoma. According to industry sources on the 16th, approximately 9 to 10 months have passed since the companies applied for reimbursement of these second-batch drugs. They are currently receiving cost-effectiveness reviews. Except for Winrevair, which received marketing authorization in July, the other two are also near approval. Among the three, Limcato was the first to apply for reimbursement in January, followed by Winrevair and Fintepla in February. Winrevair and Fintepla are being reviewed for cost-effectiveness, while Limcato is being reviewed for its clinical usefulness. Looking at the precedent set by the first batch of drugs that went through the reimbursement listing process, reimbursement for the second batch is expected early next year. As Qarziba was listed about 6 months after approval, if Winrevair, the only second batch drug approved as of now, follows a similar process, its reimbursement listing could occur as early as late this year or early next year. However, both Qarziba and Bylvay encountered hurdles at the Drug Reimbursement Evaluation Committee (DREC) stage, facing twists and turns before achieving reimbursement. Qarziba, which was approved by the Ministry of Food and Drug Safety (MFDS) in June last year, was initially deemed non-reimbursable at its first DREC review. It was subsequently granted for reimbursement in December last year after reapplying. It took approximately two years from Bylvay’s first reimbursement application in October 2023 to listing. Even at the point of its re-application last August, it had already been 14 months. This underscores the need to pass the DREC review without rejection and secure recognition of its reimbursement appropriateness, to achieve swift reimbursement listing. If a re-review process is required, reimbursement may be delayed beyond expectations. Meanwhile, the AEN linkage project is a pilot initiative aimed at enhancing access to high-priced treatments for severe diseases by shortening the period to listing of new drugs—which previously exceeded 300 days, including 120 days for MFDS (Ministry of Food and Drug Safety) approval, 150 days for HIRA (Health Insurance Review and Assessment Service) reimbursement evaluation, and 60 days for NHIS (National Health Insurance Service) drug price negotiations. During this year's National Assembly audit, the Ministry of Health and Welfare stated it would review plans to institutionalize the expedited listing system based on an analysis of the AEN pilot project's outcomes and feedback from the field.
- Policy
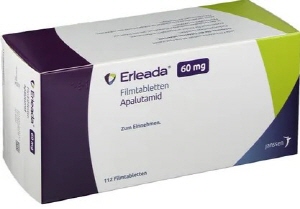
- Pricing nego for Erleada underway…last hurdle to reimb
- by Jung, Heung-Jun Nov 14, 2025 06:12am
- Janssen Korea's prostate cancer treatment Erleada (apalutamide) has entered price negotiations, the final hurdle for its reimbursement expansion in Korea. Erleada passed the Drug Reimbursement Evaluation Committee review last October and was deemed appropriate to expand reimbursement coverage to ‘high-risk non-metastatic castration-resistant prostate cancer’. According to industry sources on the 13th, the National Health Insurance Service (NHIS) is in pricing negotiations for Erleada’s reimbursement expansion. It being a drug subject to reimbursement under the risk-sharing agreement, the contract will be finalized based on the refund rate determined during negotiations. Once the price negotiations are complete, Erleada will be covered for ‘high-risk non-metastatic castration-resistant prostate cancer (nmCRPC)’ in addition to its existing coverage for ‘metastatic hormone-sensitive prostate cancer (mHSPC)’. The hormone-sensitive metastatic prostate cancer (mHSPC) indication was granted reimbursement in April 2023 as a refund-type risk-sharing agreement drug at KRW 20,045. At that time, Janssen focused its efforts on securing reimbursement for Erleada, even voluntarily lowering the price of its own prostate cancer treatment, Zytiga. It also became the first androgen receptor targeted agent (ARTA) to receive essential reimbursement at a 5% coinsurance rate. About six months later, competition intensified when Astellas Pharma Korea's Xtandi Soft Cap (enzalutamide) was added to the list as an essential reimbursement drug. Another competitor is Bayer Korea's Newbeqa, which also indicates treating ‘high-risk non-metastatic castration-resistant prostate cancer’. The company submitted a reimbursement application for Newbeqa in June this year, but the drug’s clinical benefit is still under review. With Erleada poised for reimbursement expansion, its prescription scope is also expected to broaden. Consequently, attempts to expand reimbursement for competing prostate cancer treatments are likely to follow. According to the pharmaceutical market research institution UBIST, Erleada's sales last year reached KRW 31.2 billion, a 368% increase from the previous year's KRW 6.6 billion.
- Policy
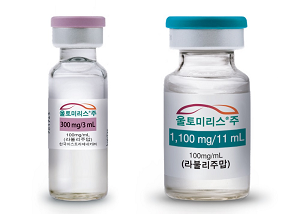
- Ultomiris gains reimbursement approval for 'NMOSD·gMG'
- by Jung, Heung-Jun Nov 14, 2025 06:11am
- Product photo of Ultomiris AstraZeneca's Ultomiris (ravulizumab) will receive reimbursement coverage for Neuromyelitis Optica Spectrum Disorder (NMOSD) this month, and its reimbursement scope will expand to generalized myasthenia gravis. Notably, the Ministry of Health and Welfare (MOHW) has requested reimbursement coverage for myasthenia gravis. Therefore, it was considered a separate agenda item for the Drug Reimbursement Evaluation Committee (DREC) held on November 6. According to industry sources, Ultomiris has passed the DREC, and it will be covered for reimbursement of 'anti-acetylcholine receptor (AchR) positive generalized myasthenia gravis (gMG)' starting December 1. Although it was not included in the meeting report disclosed by the DREC; however, it was discussed as a separate supplementary agenda. It appears that the MOHW's request for reimbursement for gMG treatments has played a role. During this year's MOHW Parliamentary Inspection, access to treatment for rare, severe diseases, including gMG, was highlighted. According to the report that Democratic Party Rep. Young-seok Seo received from the MOHW, there are no gMG medicines eligible for reimbursement. In response, the MOHW stated, "The MOHW continues to identify rare disease treatments that require urgent reimbursement coverage through seeking clinical field." It appears that the inclusion of the agenda and approval in the DREC was influenced by the critiques from the Parliamentary Inspection and the government's policy to enhance patient access. As of November 1, Ultomoris is covered by reimbursement for its indication to 'treat anti-aquaporin-4 (AQP-4) antibody-positive neuromyelitis optica spectrum disorder (NMOSD).' Eligible patients include those aged 18 or older who test positive for anti-aquaporin-4 (AQP-4) antibodies. There have been continued requests for reimbursement coverage for Ultomiris by patient organizations and petitions to the National Assembly. With the reimbursement scope expanding between November and December, more patients are likely to be prescribed in the future.
- Policy
- Pharmaceutical post-market management system to be enhanced
- by Jung, Heung-Jun Nov 14, 2025 06:11am
- The National Health Insurance Service (NHIS) plans to revise its drug price negotiation and post-market management systems next year, with a focus on stable pharmaceutical supply. The NHIS will actively enforce penalties stipulated in its regulations if pharmaceutical companies discontinue drug supply without notification in violation of agreements reached during price negotiations. This is a response to criticism raised during this year's parliamentary inspection that the agency was not adequately managing the supply obligation despite having the 'Drug Price-Reimbursement Agreement.' Se-rim Oh, Head of the Negotiation and Post-Management Unit at the Department of Drug Management.On November 11, the NHIS held a 'Drug Price Negotiation and Post-Market Management System Briefing' at its headquarters in Wonju, sharing the direction for management reinforcement with industry stakeholders for next year. The NHIS emphasized that if a company must inevitably suspend supply in violation of the agreement, it must formulate patient protection measures and consult with the NHIS beforehand. Se-rim Oh, Head of the Negotiation and Post-Management Unit at the Department of Drug Management, said, "The NHIS plans to strengthen management focusing on supply issues. If a company withdraws approval and suspends supply, it must ask pharmaceutical associations and formulate patient protection measures." Starting next year, the NHIS will actively consider imposing penalties on companies that violate their supply obligations, based on a fixed formula. The current formula sets the penalty amount per day of violation as: "(Previous Year's Annual Claim Amount of the Violating Drug) X 1/n X1/365X50%." Furthermore, companies will be required to submit data to the NHIS within 40 days of the end of each quarter to verify the fulfillment of their supply obligation (including monthly production volume, import volume, requested supply volume, and actual supply volume). The existing clause requires a company to pay a KRW 1 million fine for failure to mee these requirements. This is in response to criticism during the parliamentary inspection that the clause was not being enforced. The NHIS plans to actively enforce this rule. Oh explained, "If fulfilling the agreement is difficult, you must communicate and consult with the NHIS beforehand. Some companies have already agreed to the penalty clause after prior consultation due to the circumstances of their contract manufacturers." Oh added, "Companies failing to submit (data) are supposed to pay KRW 1 million, but this hasn't been enforced. The NHIS is aware of this. Please comply with it going forward." Eliminating reporting requirements for new efficacy and effectiveness...will review streamlining the requirement to report dosage The current obligation for companies to report any additional efficacy and effectiveness secured overseas after signing a contract with the NHIS will be exempted starting in the first half of next year. Reporting obligations when adding a new dosage will also be streamlined. Instead, if the domestic introduction of the new strength is necessary, the details will be stated in the agreement. Oh said, "The agreement includes the obligation to report when an indication is added. We are reviewing the exemption of this reporting obligation starting in the first half of next year," and added, "We are also considering simplifying the reporting obligation for adding new dosage strengths. However, if domestic introduction of a strength already listed for overseas reimbursement is necessary, we plan to address this by explicitly stating the details in the agreement. We will be gathering opinions on this matter." The NHIS is also considering the partial disclosure of information regarding Risk-Sharing Agreement (RSA) drugs. They are currently conducting consultations on disclosing a list of drugs subject to the refund-type RSA. Oh said, "If a reimbursement decision application is made to HIRA, we will ensure that information about the refund rate can be provided. However, a non-disclosure agreement must be signed," and added, "We are also conducting consultations on publicly disclosing the list of drugs subject to the refund-type RSA." PVA 'one-time refunds' will be allowed temporarily next year...new guidelines for negotiaing scope expansion will be established Hae-hee Moon, Head of the Volume Management Unit at the Department of Drug Management.The NHIS will also improve its drug utilization management plan next year. The agency plans to restrict the operation of the "one-time refund" mechanism, which was implemented during the COVID-19 pandemic, and establish new guidelines for negotiating the expansion of a drug's usage scope. Hae-hee Moon, Head of the Volume Management Unit at the Department of Drug Management, said, "We plan to limit the products eligible for the one-time refund contract starting next year. We are currently discussing this with the Ministry of Health and Welfare (MOHW). We plan to gather opinions from the pharmaceutical association afterward." Moon explained, "We are also preparing guidelines due to the need for written instructions in the process of negotiating the expansion of usage scope. We plan to conduct consultations this week and will refine these guidelines next year." The NHIS is also reviewing plans for introducing a dual pricing system with the MOHW. Moon said, "There is a request from pharmaceutical companies to introduce a dual pricing system due to the U.S. MFN policy. We are reviewing system improvements and implementation plans with the MOHW." Revision of price cap adjustment guidelines...separate adjustments for emergency imported drugs Hyung-min Kim, Head of the New Drug Management Department.The guidelines for adjusting the ceiling price during drug price negotiations will also be partially revised. The consideration for the estimated claim amount, which was previously based on the 'claim volume and growth rate of the negotiated drug over the past 3 to 5 years,' will be changed to the 'claim amount generated by the negotiated drug over the past 3 to 5 years.' Furthermore, a clause will be added to ensure that drugs urgently imported through the Korea Orphan and Essential Drug Center (KODC) will have a separate price adjustment procedure. In addition, the clause specifying 'when there is a need for public healthcare' as an exception to the three-year limit on price adjustment applications after the initial adjustment will be further specified. Hyung-min Kim, Head of the New Drug Management Department, explained, "We will define the need for public healthcare as a request for cooperation from central administrative agencies, and revise the regulation to require the Pharmaceutical Benefit Evaluation Committee to notify the NHIS of a price readjustment application," and added, "A clause will be inserted requiring measures such as refunds if the contract falls short of the mandatory production volume agreement," emphasizing the ministry's commitment to stable supply.
- Policy
- MOHW and MOJ oppose legislating mandatory INN prescribing
- by Lee, Jeong-Hwan Nov 13, 2025 06:08am
- The Ministry of Health and Welfare and the Ministry of Justice have expressed reluctance toward a bill that would only partially mandate and enforce physician international non-proprietary name (INN) prescribing for government-designated drugs with unstable supply. Despite this being President Jae-Myung Lee’s presidential election pledge and national policy task to eliminate public inconvenience and confusion over recurring drug shortages, the Ministry of Health and Welfare and the Ministry of Justice have issued a ‘careful review’ opinion. They cited the ongoing conflict between medical and pharmaceutical groups and emphasized the need to gather broader public consensus from patients (medical consumers) and society before moving forward. The Ministry of Justice specifically expressed concern that mandating INN prescribing for drugs designated by the Ministry of Health and Welfare as having unstable supply, instead of brand names, could eliminate pharmacists' obligation to inform patients—unlike in cases of substitution dispensing—thereby restricting patients' right to know and potentially posing significant risks to public health. It also added that the post-prescription accountability remains unclear. This conclusion stems from reviewing the opinions submitted on the 11th by the Ministry of Health and Welfare and the Ministry of Justice regarding the bill mandating INN prescribing for drugs with unstable supply (partial amendments to the Medical Service Act and the Pharmaceutical Affairs Act), proposed by Representative Jong-tae Jang of the Democratic Party of Korea. Rep. Jang's bill mandates that when prescribing drugs designated as having unstable supply under the Pharmaceutical Affairs Act, the active ingredient name must be written instead of the brand name, as an exception to the current law requiring the brand name on prescriptions. The bill also includes penalty provisions stipulating that violating the active ingredient name prescription requirement for supply-unstable drugs is punishable by up to one year of imprisonment or a KRW 10 million fine for noncompliance. Ministry of Health and Welfare·Ministry of Justice “Careful Review” Both the Ministry of Health and Welfare and the Ministry of Justice, the main ministries responsible for the bill, submitted opinions calling for careful review. Their view is that caution must be exercised in legislation, separate from the fact that it is a presidential election pledge and a national policy task of the Lee administration. The Ministry of Health and Welfare expressed sympathy with the intent of introducing INN prescribing, which aims to ensure continuity of patient treatment during drug supply disruptions like shortages. However, it stated that disagreements between doctors' and pharmacists' professional organizations regarding the safety and efficacy of INN prescribing must be taken into account. This is the fundamental response the Ministry has repeatedly given in past statements regarding INN prescribing. The Ministry further stated that criteria for defining drug supply instability, measures to ensure safety and efficacy of INN prescribing, and effective implementation methods for INN prescribing must first be reviewed. The intent is to further discuss whether to introduce mandatory INN prescribing for physicians or adopt an indirect approach, such as providing incentives to prescribers who use INN prescribing. The Ministry also raised concerns about the bill's penalty provisions. It cautioned that criminalizing doctors by imposing criminal penalties for not following INN prescribing when prescribing drugs with unstable supply is an approach that requires careful consideration. The Ministry of Justice provided more specific reasons for playing caution regarding the bill. First, it stated that designating a drug as having an unstable supply would force doctors to prescribe by INN instead of brand name, which would have the effect of broadly permitting substitution dispensing regulated by the Pharmaceutical Affairs Act. The current Pharmaceutical Affairs Act’s substitution dispensing provisions require informing the patient of the substitution and obtaining prior consent from the prescribing physician or providing post-notification within one day. The Ministry of Justice opined that under Rep Jong-tae Jang’s bill, the obligation to inform patients would disappear for designated unstable-supply drugs, and the requirement to obtain prior physician consent or provide post-notification would also vanish, potentially creating problems. The Ministry pointed out, “There would be no need to inform patients, and as notification or prior consent from physicians is not required, this may potentially be a significant risk to public health. This restricts patients' right to know and also leaves unclear whether prescribers would bear responsibility after the fact.” KMA·KHA “Oppose”…KPA “Favor” The Korean Medical Association (KMA) and the Korean Hospital Association (KHA) voiced strong opposition. The KMA argued that mandating INN prescriptions is excessive legislation, as it cannot be a fundamental solution to unstable drug supply issues, yet it stipulates penalties of up to 1 year in prison or fines of up to KRW 10 million for non-compliance. It further argued that forcing doctors to INN prescribe drugs with unstable supply without considering patient conditions disregards public health rights. The KMA also contended that mandating INN prescribing undermines patient safety and treatment continuity, completely infringes on physicians' prescribing rights, and violates the fundamental principle of separating medical and pharmaceutical practices. The KMA stated, “A physician's diagnosis and prescription constitute a professional medical act that comprehensively considers individual patient characteristics, including disease status, underlying conditions, presence of drug allergies, past drug reactions, and ease of administration. When prescribing drugs, physicians select specific products—including the most suitable dosage form, strength, excipients, and coating technology—based on the specific disease and patient characteristics. Forcing INN prescribing hinders patient treatment, infringes on physicians' prescribing rights, and undermines the principle of separation of medical and pharmaceutical services." The KHA also opposed the measure, stating that instability in drug supply arises from multiple causes, such as raw material shortages, production plant issues, distribution problems, pricing, and increased demand due to specific disease outbreaks. KHA argued that legally mandating INN prescribing would be insufficient to resolve the problem and could potentially infringe on physicians' prescribing rights. The KHA emphasized that establishing comprehensive national policies would be necessary to create an environment enabling a stable drug supply. It further pointed out that penalizing non-compliance with INN prescribing, even when physicians may unknowingly prescribe by brand name due to difficulties in quickly and accurately assessing drug supply situations, violates the principle of proportionality. The KHA stated, “Long-term, fundamental measures must be established, such as creating a management system to prevent supply instability in advance. The penalties are excessive sanctions compared to other violations subject to the same penalties under current law, relative to the risk and illegality of the act.” The Korean Pharmaceutical Association (KPA) countered that INN prescribing is the most effective solution to respond swiftly to drug supply instability issues. The association's rationale for supporting the bill is that this legislation will reduce the social and economic waste caused by drug shortages and enable timely dispensing and medication services for patients. Specifically, the association argued that it is irrational for pharmacies to wait solely for a specific brand (product) to arrive when equivalent generic alternatives are available. It also expects the bill to alleviate the burden on pharmacies, which must stock medications from multiple pharmaceutical companies to accommodate individual prescriptions from different medical institutions, even for the same active ingredient. The KPA stated that Representative Jang’s bill does not contradict the existing legal framework, as the Medical Service Act and its enforcement regulations already permit INN prescribing. It also argued that distrusting or claiming differing efficacy for generics approved and registered by the Ministry of Food and Drug Safety (MFDS) is unscientific. The KPA emphasized, “When an INN prescription is issued, even if the supply of a specific pharmaceutical company's drug is unstable, the same active ingredient medication needed by the patient can be dispensed on time, preventing treatment gaps. This allows patients to obtain their prescribed medication anywhere, strengthening public access to medicines and their right of choice.” Welfare Committee expert committee presents divergent views on generic efficacy equivalence The National Assembly Health and Welfare Committee’s expert committee acknowledged that legislation has a valid aspect, as mandatory INN prescribing could help stabilize drug supply. However, it also presented points to consider during legislation. Unlike substitution dispensing, INN prescribing eliminates the need for prior physician consent or post-dispensing notification by pharmacists. In this sense, the expert committee noted that the differing opinions surrounding the therapeutic equivalence of identical-ingredient drugs (generics) must be examined. The expert committee stated, “Those affirming the therapeutic equivalence of identical-ingredient drugs maintain that generics undergo the Ministry of Food and Drug Safety's strict approval and review system, thereby ensuring recognized bioequivalence. “Conversely, those who deny equivalence argue that even with the same active ingredient, differences exist between products in formulation, dosage, excipients, coating technology, etc. They contend that when patients take a different product than before, it increases the potential for drug side effects and may lead to uncertainty in treatment efficacy.” The expert committee also pointed out that the Ministry of Health and Welfare recently clarified the requirement for post-substitution notification. It noted that the revised Pharmaceutical Affairs Act, which aims to enhance information sharing between doctors and pharmacists, passed the National Assembly, was submitted to the government, and was promulgated. Finally, it stated that the penalty clause, which imposes imprisonment for up to one year or a fine of up to KRW 10 million for violations, should also be examined for potential excessiveness. The expert committee stated, “Recently, concerns have been raised that imposing excessive penalties for administrative violations burdens criminal justice agencies and creates a large number of citizens with criminal records. Consequently, legislative reforms are underway to convert fines for minor administrative law violations into administrative penalties. In the exceptional situation where INN prescribing is mandated for unstable supply drugs, it is necessary to examine whether imposing criminal penalties for prescribing by brand name due to lack of awareness constitutes excessive punishment for violating administrative order.”
- Policy
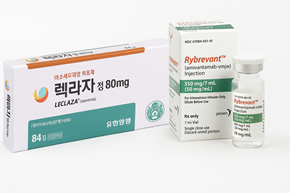
- Yuhan–Janssen join forces to tackle lung cancer
- by Jung, Heung-Jun Nov 12, 2025 06:19am
- As Yuhan Corporation and Janssen Korea join forces to co-promote their lung cancer treatments, securing reimbursement for the combination therapy has emerged as the next major hurdle to fully realize their market synergy. Janssen Korea’s non-small-cell lung cancer drug Rybrevant (amivantamab) was granted reimbursement as monotherapy by the Cancer Disease Deliberation Committee (CDDC) in September, but its combination with carboplatin or lazertinib did not receive reimbursement approval. According to industry sources on the 10th, Janssen Korea has submitted a request for reconsideration to the CDDC regarding the unestablished reimbursement criteria. At that meeting, Rybrevant’s reimbursement was not established for use in ▲combination with carboplatin and pemetrexed as first-line therapy for adults with locally advanced or metastatic EGFR exon 20 insertion-positive NSCLC, and in ▲combination with lazertinib for adults with EGFR exon 19 deletion or exon 21 (L858R) substitution mutation-positive NSCLC. However, Janssen Korea applied for a reimbursement re-evaluation for the combination therapy with carboplatin and pemetrexed 3 weeks after the CDDC decision. A cost-effectiveness review is underway for the re-evaluation. Also, with its combined use with Leclaza, which is also accumulating clinical evidence, the company’s attempts for reimbursement listing are expected to continue. Recently, the National Comprehensive Cancer Network (NCCN) also prioritized the combination therapy of Leclaza + Rybrevant as a first-line treatment for EGFR-positive non-small cell lung cancer. Given Rybrevant’s high price, public demand for its reimbursement had been strong, with a national petition for reimbursement gathering over 50,000 signatures, leading to formal discussion in the National Assembly earlier this year. During the latest NA audit, multiple lawmakers again raised questions about its reimbursement timeline. Rep. Nam-Hee Kim (Democratic Party of Korea) specifically urged the Ministry of Health and Welfare (MOHW) to establish reimbursement criteria for first-line treatment and expedite insurance coverage. In response, the Ministry stated, “The pharmaceutical company has applied for a re-evaluation of reimbursement criteria for the first-line treatment indication that had not been set the last time. We plan to proceed with the process and determine reimbursement eligibility.” Meanwhile, Janssen Korea, the domestic pharmaceutical subsidiary of Johnson & Johnson, announced on the 10th a joint promotion plan with Yuhan Corporation for the combination therapy of ‘Leclaza (lazertinib)’ + ‘Rybrevant (amivantamab)’.
- Policy
- NA requests rapid reimb listing for diabetes drug Mounjaro
- by Jung, Heung-Jun Nov 11, 2025 06:08am
- The Ministry of Health and Welfare (MOHW) announced that it will reflect the results of the October expert advisory meeting in reimbursement criteria for Mounjaro (tirzepatide), Eli Lilly’s dual GIP/GLP-1 receptor agonist, following growing calls for its rapid inclusion under the National Health Insurance for type 2 diabetes. The ministry also stated it is working to ensure the smooth reimbursement process for Ozempic (semaglutide), Novo Nordisk’s GLP-1 receptor agonist, which was reviewed by the Drug Reimbursement Evaluation Committee in October. On the 10th, Miae Kim, a lawmaker from the People Power Party, inquired about the current status of the reimbursement application process for Mounjaro for type 2 diabetes during a written inquiry session for the Ministry of Health and Welfare's comprehensive audit. She also questioned the plans for utilizing the expedited listing system, which reflects international drug prices and reimbursement cases. According to the Ministry of Health and Welfare, Mounjaro applied for reimbursement approval in March last year and was discussed by the Drug Reimbursement Criteria Subcommittee in May of the same year. Subsequent evaluations on its reimbursement adequacy were conducted in August and December 2023, and March and June 2024 by the Cost-Effectiveness Evaluation Subcommittee. The Ministry explained, “The company applied for reimbursement of Mounjaro in March last year and is currently undergoing a review on its reimbursement adequacy. The drug is being reviewed in accordance with HIRA regulations, referencing factors such as its listing status in other countries, listed prices, and coverage criteria.” It added, “To ensure appropriate use of the GLP-1 agonists Mounjaro and Ozempic for diabetes patients, HIRA held an expert advisory meeting last October to discuss patient selection criteria and evaluation methods. The findings will be reflected in the drug's reimbursement criteria.” Rep. Seo Mi-hwa of the Democratic Party of Korea also inquired about the Ministry's stance on reviewing insurance coverage for new drugs to strengthen diabetes management. The Ministry responded, “We agree that the health insurance coverage of new diabetes drugs should be actively reviewed. Currently, GLP-1 receptor agonists Ozempic and Mounjaro are undergoing the listing process. We will closely monitor to ensure the listing process proceeds smoothly and continue our efforts to improve treatment accessibility.”
- Policy
- Cancer & orphan drugs apply for reimb within 1 week of apv
- by Jung, Heung-Jun Nov 10, 2025 06:10am
- Anticancer drugs and rare disease drugs that were approved in Korea this year are racing to apply for reimbursement as quickly as within a week. Six rare/serious disease treatments approved by the Ministry of Food and Drug Safety this year are undergoing reimbursement review, excluding those subject to the approval-evaluation-negotiation system. According to industry sources on the 7th, multiple new drugs, including those designated under the Global Innovative product on Fast-Track (GIFT) scheme, are applying for reimbursement quickly after approval. Takeda Korea’s Fruzaqla (fruquintinib), which was approved in March for metastatic colorectal cancer, was submitted for reimbursement last month and is currently being assessed for clinical benefit. The drug, designated GIFT No. 20, was specifically requested for expedited coverage during this year’s NA MOHW audit as a third-line therapy for metastatic colorectal cancer. Qalsody (tofersen), Biogen’s amyotrophic lateral sclerosis (ALS) treatment and GIFT No. 31, received approval on August 20 and was immediately filed for reimbursement to expand its prescription. Imdelltra (tarlatamab), Amgen Korea’s small-cell lung-cancer drug (GIFT No. 25), was approved on May 30 and submitted for reimbursement on August 28. Augtyro (repoterrectinib), Bristol Myers Squibb’s therapy for metastatic non-small-cell lung cancer and solid tumors, followed a similar path — approved in June, filed 2 months later. Gilead Sciences’ Yescarta (axicabtagene ciloleucel), a CAR-T therapy for diffuse large B-cell lymphoma, and Janssen Korea’s Opsynvi (macitentan + tadalafil) for pulmonary arterial hypertension, both received approval in July and August, respectively, and each applied for reimbursement within just 1 week of approval. Opsynvi is now undergoing a cost-effectiveness evaluation. Industry analysts attribute this accelerated pace partly to the current administration’s policy direction under President Jae-myung Lee, which emphasizes improving patient access to orphan and severe-disease treatments. However, even with early reimbursement filings, such drugs typically face lengthy review cycles as significant time is required for clinical utility and cost-effectiveness reviews. Even if they clear the threshold of proof, a protracted drug price negotiation process remains. Currently, 33 drugs that applied for reimbursement last year and this year remain under review, suggesting fierce behind-the-scenes competition among drugs for priority listing.