- LOGIN
- MemberShip
- 2026-03-12 00:35:29
- Policy
- Daewoong’s P-CAB Fexuclue completes first PVA
- by Lee, Tak-Sun Feb 17, 2025 05:53am
- Daewoong Pharmaceutical's new drug for gastroesophageal reflux disease, ‘Fexuclue,' has agreed to complete negotiations through the price-volume agreement system with the National Health Insurance Service for the first time since its reimbursement listing. Before negotiations, Daewoong Pharmaceutical was known to have wanted to apply the reimbursement system rather than a price cut, so the outcome of the negotiations is drawing attention. According to industry sources on the 16th, Daewoong Pharmaceutical's ‘Fexuclue Tab 10, 40mg’ and Daewoong Bio's ‘We Cab Tab 10, 40 mg.’ which contain identical ingredients, have completed price-volume agreement negotiations. The PVA system allows the NHIS and pharmaceutical companies to negotiate and reduce the maximum insurance price of drugs with increased usage by up to 12.5% this year. The aim is to save Korea’s national health insurance finances. The type of negotiation applied to Fexuclue this time is Type A, which is carried out when the amount of claims in the same product group with the expected claims amount agreed with the NHIS increases by 30% of the expected claims amount. Fexuclue, which was listed for reimbursement in July 2022, recorded a prescription volume of KRW 12.9 billion in the first year of its release based on UBIST and then grew exponentially to record a prescription volume of KRW 78.8 billion last year (2024). In particular, the analysis shows that the product’s growth accelerated after signing a copromotion agreement with Chong Kun Dang in April last year In response, the NHIS selected Fexuclue as a drug subject to PVA monitoring in the fourth quarter of last year, and negotiations began in early December of that year upon the Ministry of Health and Welfare’s order. Before negotiations, it was reported that Daewoong Pharmaceutical wanted to apply the refund-type agreement like its competitor ‘K-CAB (HK Inno.N).’ The refund-type agreement is a system that maintains the maximum insurance price of a drug while returning the difference to the National Health Insurance Service. When exporting the drug overseas, this can be advantageous as the drug’s listed price is higher than the actual price. Currently, K-CAB is the only product that has signed a refund-type PVA system. In 2015, Boryung’s hypertension drug Kanarb entered into a refund-type agreement, but in 2018, it chose to lower the maximum insurance price instead of extending the contract. On the other hand, K-CAB entered into a refund-type agreement in 2021 and extended the agreement at the end of last year. As a result, its ceiling price of KRW 1,300 per tablet was maintained when it was first listed in 2019. Fexuclue is listed at KRW 939, which is 70% of the price of K-CAB. If the ceiling price is lowered through the PVA, the gap between K-CAB and Fexuclue will widen further. However, the relatively low price of the drug may be advantageous in sales competition between products, so it is interesting to see what kind of agreement Daewoong and the NHIS have reached. P-CAB (potassium-competitive acid blocker) class drugs like Fexuclue are characterized by a longer duration of efficacy due to their longer half-life than PPIs, making them effective for controlling nighttime acid secretion. It is easy to take because it only needs to be taken once a day, regardless of meal intake, and its market size has been rapidly increasing. Currently, in addition to K-CAB and Fexuclue, ‘Ja Q Bo (Onconic Therapeutics, a subsidiary of Jeil Pharmaceutical),’ a new domestic drug with the same mechanism of action (P-CAB), has also been listed for reimbursement, making it a three-way competition.
- Policy
- Vivozon’s VVZ-2471 patent registered in China
- by Lee, Jeong-Hwan Feb 14, 2025 05:58am
- On the 23rd, Vivozon (CEO: Doo-hyun Lee), a company specializing in the development of innovative new drugs, announced that it completed registering the product patent for its oral non-narcotic analgesic VVZ-2471, which is being developed as a treatment for pain and addiction, and its derivatives in China. This is the company’s third patent registration abroad, following the United States and South Africa. Vivozon is also undergoing the patent registration process in other major countries. This patent contains content that ensures VVZ-2471’s exclusive rights in China and the protection of related compounds based on its superior analgesic effect and differentiated effect compared to existing compounds. VVZ-2471 is a new drug candidate discovered by the company’s proprietary multi-target drug discovery technology. It has been confirmed to have an analgesic effect as well as an effect on treating drug addiction. Vivozon is conducting clinical trials to develop it as a treatment for neuropathic pain in Korea and as a treatment for drug addiction in the United States. In particular, Vivozon received IND approval for a Phase II clinical trial for VVZ-2471 in June last year for patients with post-herpes zoster neuralgia in Korea. The company plans to widely use the 38th homegrown new drug Unafra Inj. (Opiranserin hydorochloride), which was granted marketing authorization from the Ministry of Food and Drug Safety last year, along with its oral analgesic candidate VVA-2471. “We expect the results of VVZ-2471’s Phase III clinical trial in Korea to be available within this year,” said a Vivozon representative. ”The patent registration in China recognizes the analgesic effect of VVZ-2471 in the market, where there is no non-narcotic analgesic with efficacy comparable to that of narcotic analgesics.” He also said, “Our goal is to develop VVZ-2471 as a non-narcotic oral analgesic for acute and chronic pain and lead the global analgesic market along with Unafra Inj.” Meanwhile, Vivozon is conducting a Phase II clinical trial to develop VVZ-2471 as a drug addiction treatment to address the serious social issue of addiction and abuse of narcotic analgesics (opioids) such as fentanyl in the United States. To this end, Vivozon is applying for research funds from the National Institute on Drug Abuse (NIDA) under the National Institutes of Health (NIH) and is cooperating with local drug addiction treatment experts.
- Policy
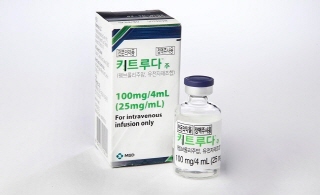
- Six of Keytruda's indications fail reimbursement
- by Lee, Tak-Sun Feb 14, 2025 05:58am
- After 5 failed attempts, MSD succeeded in receiving reimbursement standards for 11 additional indications of its immuno-oncology drug Keytruda in its 6th attempt. However, the company unfortunately has been denied reimbursement 6 indications. The six indications that have been denied reimbursement this time can only be reviewed if the company reapplies for reimbursement. On the 12th, the Health Insurance Review and Assessment Service's Cancer Disease Deliberation Committee set reimbursement standards for 11 additional indications of MSD's immuno-oncology drug Keytruda. As indications for which reimbursement standards have been set must undergo a drug price negotiation with the National Health Insurance Service after the Drug Reimbursement Evaluation Committee reviews the adequacy of their reimbursement, there are still many hurdles to overcome. Even so, the fact that the reimbursement standards for a single drug with a large number of indications were set and passed CDDC review is interpreted as a significant step in its reimbursement progress. Expanding the scope of reimbursement to 11 indications is expected to require a huge amount of health insurance finances. The 11 indications for which the salary criteria have been set this time are: ▲PD-L1 positive advanced or metastatic HER2-positive gastric cancer; ▲ advanced or metastatic HER2-negative gastric cancer; ▲ PD-L1 positive advanced or metastatic esophageal cancer ▲ MSI-H or dMMR advanced endometrial cancer; ▲ MSI-H or dMMR metastatic colorectal cancer; ▲ advanced or recurrent head and neck cancer with MSI-H or dMMR; ▲ metastatic or recurrent squamous cell carcinoma; ▲PD-L1-positive persistent, recurrent, or metastatic cervical cancer; ▲PD-L1-positive recurrent or metastatic triple-negative breast cancer ▲ MSI-H or dMMR Metastatic endometrial cancer; ▲ MSI-H or dMMR metastatic small intestine cancer; ▲ MSI-H or dMMR metstatic biliary tract cancer. MSD applied for reimbursement expansion for 13 indications in 2023 and added 4 indications last year. While the company succeeded in establishing reimbursement standards for 11 indications this time, it failed to do so for the remaining 6 indications. An official from HIRA explained, “The 11 indications for which the reimbursement criteria have been set this time have been pending for the past 2 years. The review of the financial sharing plan submitted by the pharmaceutical company has been completed, based on which the reimbursement criteria have been set during CDDC review.” “On the other hand, the results of the review showed that the 6 indications were not adequate for reimbursement without undergoing CDDC deliberations,” the official added. ”If the company applies for reimbursement again for the indications, it will be able to receive review.” The 6 indications for which the reimbursement standards have not been set are: ▲early triple-negative breast cancer, ▲adjuvant therapy after surgery for renal cell carcinoma, ▲non-invasive bladder cancer, ▲MSI-H or dMMR metastatic ovarian cancer, ▲MSI-H or dMMR metastatic pancreatic cancer, and ▲MSI-H gastric cancer. Currently, Keytruda is reimbursed for 7 indications in 4 cancer types, including as a first-line treatment for non-small cell lung cancer, melanoma, urothelial cancer, and Hodgkin's lymphoma, with annual claims amount reaching KRW 400 billion. Meanwhile, CDDC plans to reach a consensus on the need for a principle for the reimbursement of high-priced anticancer drugs and to establish detailed standards in the future, taking the review of this Keytruda as an opportunity. An official from the Health Insurance Review and Assessment Service said, “During CDDC review, there was a consensus on the need to establish a principle for the reimbursement of high-priced anticancer drugs and to review them as in some countries, such as the United States. We plan to discuss this in detail at the next CDDC meeting.”
- Policy
- MOHW "Why is HIRA concerned about substitute prescriptions?"
- by Lee, Tak-Sun Feb 14, 2025 05:58am
- The Ministry of Health and Welfare (MOHW) announced adding 'HIRA's Business Portal System' as part of the post-notification procedures for substitute prescriptions. However, an issue has been raised regarding differing opinions between the MOHW, which is responsible for the policy implementation, and the Health Insurance Review and Assessment Service (HIRA). On February 12, the MOHW team expressed discomfort regarding HIRA President Jung-Gu Kang's announcement of concerns to the press. The announcement highlighted that neither party had sufficiently discussed the agreement details during the revision of the Pharmaceutical Affairs Act guidelines and policies. The pharmacy industry deeply concerned about whether the revision can be effectively implemented given this ongoing dispute. HIRA, "We disagree with the inclusion of post-notification procedure…MOHW decided the portal service" According to industry sources, the MOHW was solely responsible deciding to include 'HIRA's Business Portal System' in the revised details of the Pharmaceutical Affairs Act guidelines and policies regarding post-notification procedures for substitute prescriptions. The HIRA did not mutually agree. It has been reported that HIRA expressed negative opinions towards utilizing either the DUR or the business portal system for post-notification procedures for substitute prescriptions. Before the notification of the implementation of the revision, the administrative team expressed concern about invading the system's intended role and potential incidents related to pharmaceuticals due to doctors not being aware of the updates. However, during the exchange of opinions, it has been reported that the party suggested the time it takes to develop a new system. After the MOHW announced the implementation of the revision to the Pharmaceutical Affairs Act on January 21, HIRA has not provided further opinion to the MOHW. The current post-notification procedure for substitute prescriptions is limited to 'telephone, fax, or computer network.' The MOHW announced the implementation of revision to the Pharmaceutical Affairs Act guidelines and policies on January 21 with details of including the 'HIRA's Business Portal System' as part of the notification method. Amid this situation, the HIRA president, who will be responsible for running the portal for substitute prescriptions, expressed concerns to the press. During the press conference the other day, Kang said, "Substitute prescriptions with the same active ingredient will not present significant issues for pharmaceuticals. However, we must be cautious because patients may have varying sensitivity for certain medicines." Kang added, "If the HIRA's portal is used, doctors must individually access the system and check the change. Thus they may be aware of the substitute prescription later or not at all in some cases." Kang also added, "We need to review ways to inform doctors about substitute prescription incidence immediately." MOHW, "Difficult to understand Kang's concerns…an agreement from doctors on the post-notification procedure is not necessary" Regarding this matter, the MOHW responds that it is difficult to understand why Kang announced a negative opinion towards the inclusion of the business portal for the post-notification procedure as part of the revision. Since the revision entails simply adding the business portal to existing methods, including phones, fax, and computer networks, the MOHW reportedly to disagree with Kang's argument that doctors' awareness and confirmation of substitute prescriptions will be delayed. During the meeting with the Korea Special Press Conference, a MOHW Bureau of Policy Planning representative said, "We did not revise the details to the guidelines to rely on the business portal system solely. We intended to add more notification channels." He added, "The intention is to add the business portal system to the existing methods, such as phones and fax, so it is difficult to understand why Kang expresses concerns." The representative added, "(If HIRA's system is used) Doctors checking the change could be delayed, but this is not intended to ask permission, but it is a notification. Adding another notification channel won't delay the check." He explained, "Technically, MOHW's adding a post-notification system to HIRA's portal is intended to support doctors and pharmacists' mutual access, and the HIRA's involvement is none." "If it (post-notification service for substitute prescriptions on the business portal) were HIRA's responsibility, Kang's concern would be important, but the HIRA does not play any role in this procedure. HIRA may be running the business portal system. However, MOHW is simply establishing the channel, and the HIRA is not responsible for mutual communication between doctors and pharmacists," the representative emphasized. "Because (HIRA) is a third party allowing the space, and does not play a specific role in the portal system, we are unclear about why (Kang) expresses opinions or whether it's his responsibility," the representative added.
- Policy
- P3T for Sanofi’s Itepekimab in rhinosinusitis approved
- by Lee, Hye-Kyung Feb 12, 2025 06:13am
- Sanofi Aventis' biological drug 'Itepekimab' is entering Phase III trial in Korea to secure an indication for chronic rhinosinusitis following chronic obstructive pulmonary disease (COPD). On the 10th, the Ministry of Food and Drug Safety approved Sanofi’s 52-week Phase III randomized, double-blind, placebo-controlled, parallel-group clinical trial to investigate the efficacy, safety, and tolerability of Itepekimab in adult subjects with chronic rhinosinusitis with uncontrolled nasal polyps. Chronic rhinosinusitis is defined as inflammation of the nasal cavity and paranasal sinuses that lasts for 12 weeks or longer. Some doctors also describe inflammation of the nose and sinuses when nasal inflammation (rhinitis) is present. In Korea, it is said that about 8.4% of the total population has sinusitis, and the prevalence of chronic rhinosinusitis with polyps is 2.6%. With the approval, the number of clinical trials being conducted for Itepekimab since 2021 in Korea has increased to four in total. Since 2021, two Phase III clinical trials have been underway for COPD patients, and Phase II and III trials are being conducted simultaneously for chronic rhinosinusitis patients. Itepekimab is a monoclonal antibody that targets interleukin (IL)-33, an inflammatory cytokine. Autoimmune diseases associated with IL-33 include allergic diseases, atopic allergies, asthma, rheumatoid arthritis, multiple sclerosis, and systemic lupus erythematosus, and it plays an important role in the functioning of the immune system. Sanofi is developing Itepekimab as the next-generation drug to succeed Dupixent (dupilumab). Dupixent is the first biological agent that targets the signaling of interleukin (IL)-4 and IL-13, which are the main causative agents of type 2 inflammation and has been approved as an atopic dermatitis treatment in several countries, including South Korea, the United States, Canada, Europe, Japan, and Australia. It has since expanded its indications to include patients with moderate-to-severe asthma, nodular rash, COPD, and eosinophilic esophagitis. Currently, Sanofi is seeking to further obtain indications for Dupixent for chronic idiopathic urticaria (CSU), chronic pruritus of unknown origin (CPUO), and bullous pemphigoid (BP).
- Policy
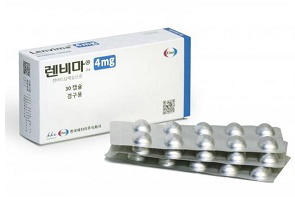
- Boryung’s Lenvima generic approved in Korea
- by Lee, Hye-Kyung Feb 11, 2025 06:03am
- Boryung has received approval for a generic version of the liver cancer treatment 'Lenvima Cap (lenvatinib).' It received approval as an incrementally modified drug (new salt version) and added a 12 mg dose, which is not available with the original drug. The Ministry of Food and Drug Safety (MFDS) approved three items on the 6th, including 4 mg, 10 g, and 12 mg doses of Lenvanib Cap (Lenvatinib Mesylate Dimethyl Sulfoxide) from Boryung. Lenvanib was approved by adopting a salt modification strategy, adding dimethyl sulfoxide (DMSO) to the mesylate of the original main ingredient, lenvatinib mesylate. Like the original, Lenvanib has 4 indications: ▲ for the treatment of locally recurrent or metastatic progressive differentiated thyroid cancer that is not responsive to radioactive iodine ▲ as a first-line treatment for patients with unresectable hepatocellular carcinoma ▲ in combination with pembrolizumab, for the treatment of patients with advanced endometrial carcinoma (EC) that is not microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) who have disease progression following prior systemic therapy in any setting and are not candidates for curative surgery or radiation, and ▲ in combination with pembrolizumab, for the first line treatment of adult patients with advanced renal cell carcinoma. The new 12 mg dose, which is not available with the original, can be administered to patients weighing more than 60 kg, increasing patient convenience. However, in the case of Boryung, the actual launch date for the drug is unknown as the company has been in a patent dispute for Lenvima for over 2 years. Lenvima has six patents, including a substance patent expiring in April 2025, ▲ a use expiring in March 2028, ▲ a crystalline form patent expiring in June 2028, ▲ a formulation patent expiring in March 2031, ▲ a composition patent expiring in August 2035, and ▲ a use patent not listed, expiring in December 2035. In the Lenvima composition patent dispute, Boryung won the first trial but is awaiting the decision of the Patent Court following an appeal by Eisai. When Eisai registered a new use patent on its use in combination with Keytruda for advanced endometrial cancer in April last year and in combination with Keytruda for first-line treatment of advanced renal cell carcinoma, Boryung filed a request for invalidation and a request for confirmation of the passive scope of rights for the new unlisted patent. In order for Boryung to launch its Lenvanib, it must first win the trial to invalidate the patent for its intended use, which it filed in November 2022. In addition, it must win the second trial that will be held as a result of Eisai's appeal. In addition, it must circumvent or invalidate the newly registered Eisai’s patent. Meanwhile, according to the market research institution UBIST, sales of Lenvima last year were KRW 12.8 billion.
- Policy
- KAPO ‘CDDC should pass Keytruda’s reimb extension agenda’
- by Lee, Tak-Sun Feb 11, 2025 06:02am
- A patient group has called for the immediate expansion of the coverage of the immuno-oncology drug 'Keytruda'. With the National Health Insurance Service's Cancer Disease Deliberation Committee scheduled to meet on the 12th, the group is asking for the establishment of reimbursement standards to strengthen patient access to treatment. In a statement issued on the 10th, the Korea Alliance of Patients Organization (KAPO) said, “Immuno-oncology drugs are innovative treatments that have changed the paradigm of cancer treatment. While conventional anticancer drugs directly attack cancer cells, the immuno-oncology drugs activate the patient's immune system to eliminate cancer cells on their own, providing new treatment opportunities for cancer patients who have experienced limitations with existing treatments.” Keytruda (pembrolizumab) is a representative immuno-oncology drug that was first approved by the US Food and Drug Administration (FDA) in September 2014. It was approved by the European Medicines Agency (EMA) in July 2015, and currently has 31 and 39 approved indications, respectively. In Korea, it was first approved by the Ministry of Food and Drug Safety on March 6, 2015, for the treatment of inoperable or metastatic melanoma. Since then, its indications have been expanded, and a total of 34 indications have been approved for 16 different types of cancer. Although Keytruda has many indications, only a few are covered by national health insurance. As KAPO pointed out, only 7 indications are covered by national health insurance for 4 types of cancer: non-small cell lung cancer, Hodgkin's lymphoma, melanoma, and urothelial carcinoma. This is significantly less than what is covered in the UK (19), Canada (18), and Australia (14). Currently, discussions on the expansion of the coverage of Keytruda in Korea are underway, starting with a request for the expansion of coverage for 13 indications in 2023, followed by an additional 4 indications in 2024, for a total of 17 indications. KAPO pointed out, “While discussions on reimbursement extensions have been delayed for the second year, patients are missing out on appropriate treatment opportunities. The damage that patients suffered due to the delay in expanding the coverage of the first-line treatment of non-small cell lung cancer in 2017 should not be repeated.” He also urged, “Treatments should be a door of hope for the patients. However, the government and pharmaceutical companies are now making patients wait behind closed doors. The government and pharmaceutical companies should not delay expanding coverage of Keytruda any longer on the issue of sharing the financial burden.” KAPO said, “We urge the Cancer Disease Deliberation Committee to pass Keytruda’s reimbursement extension proposal on the 12th, at the first CDDC meeting in 2025. The drug reimbursement adequacy evaluation by the Drug Reimbursement Evaluation Committee and the drug pricing negotiations between the National Health Insurance Service and pharmaceutical companies should be carried out promptly thereafter. The government and pharmaceutical companies should no longer postpone their responsibilities.”
- Policy
- Pricing negotiations complete for Pfizer’s Vyndamax
- by Lee, Tak-Sun Feb 10, 2025 05:50am
- Pfizer has completed pricing negotiations with the National Health Insurance Service for its cardiomyopathy drug, Vyndamax Cap (tafamidis). As a result, the drug is soon expected to be listed for reimbursement benefits in Korea. Four and a half years after its approval in August 2020, the drug is finally being included in Korea’s health insurance system. According to industry sources on the 7th, the National Health Insurance Service and Pfizer Korea recently agreed on the insurance price of Vyndamax Cap 61 mg, for which the pricing negotiations began in December last year. The NHIS and Pfizer reportedly reached an agreement through a risk-sharing agreement. Vyndamax has gained attention as the only treatment option for ATTR amyloidosis with cardiomyopathy (ATTR-CM). ATTR-CM is a devastating disease with a median survival of 2 to 3.5 years without adequate treatment but has been poorly treated, either because it is mistaken for simple heart failure or because there are no other treatments available. In this area with a dire need, Vyndamax has demonstrated its efficacy in reducing the incidence of cardiovascular events and improving the 6-minute walk test in CM patients through the Phase 3 ATTR-ACT study. Although it is the only treatment for the disease, its approval process has not been smooth. After receiving domestic approval in 2021, the company began to pursue reimbursement in earnest but was stuck in HIRA’s evaluation phase. In April 2022, the drug failed to pass HIRA’s Drug Reimbursement Subcommittee in April 2022, and then was presented to the Drug Reimbursement Evaluation Committee (DREC) review in April 2023, the last stage of HIRA’s review, but was not deemed adequate for reimbursement. However, in October of last year, it finally passed the DREC review and has been negotiating with the NHIS since early December by the Ministry of Health and Welfare's negotiation order. By completing negotiations with the NHIS, the reimbursement listing process has now been virtually completed. The only remaining procedures are the report to the Ministry of Health and Welfare’s Health Insurance Policy Deliberation Committee (HIPDC) and notification. It is expected that the report will be presented to the HIPDC as early as this month and will be listed for reimbursement on March 1. As the only treatment for ATTR-CM, Vyndamax’s reimbursement will provide patients with much better access to treatment. As of 2021, there are 75 patients with ATTR-CM in Korea.
- Policy
- HIRA to develop RWE guidelines for post-listing drug evals
- by Lee, Tak-Sun Feb 07, 2025 05:51am
- The Health Insurance Review and Assessment Service (HIRA) has begun developing real-world evidence (RWE) guidelines for the evaluation of drugs listed under the post-listing drug control condition, with the goal of resolving uncertainties in the reimbursement decision-making process On the 5th, HIRA announced a request for proposals for research on guidelines for generating real-world evidence (RWE) for drug performance evaluations.” “Recently listed high-priced treatments for serious diseases have high uncertainty in terms of the level of evidence, but they are listed under the condition of post-listing control to improve patient access,” said the agency. ”Real-world data (RWD) and real-world evidence (RWE), which are the main sources of drug performance evaluation for post-listing control, are at risk of bias, so it is necessary to improve the reliability of RWE generations.” Drugs listed under the post-listing control condition are those that have only short-term, single-arm, small-patient trial results or have been exempted from submitting pharmacoeconomic evaluation data. HIRA plans to conduct post-listing evaluations to resolve the uncertainty of reimbursement of such drugs. RWE data is used for this purpose, but there is an urgent need for guidelines to improve reliability. Major countries such as the United Kingdom and Canada have developed and utilized their own RWE guidelines to better accommodate their decision-making process. The study aims to ▲ review the current status of RWE utilization for drug performance evaluations make recommendations and ▲ prepare guidelines for generating real-world evidence (RWE) for drug performance evaluations. The study is expected to improve the transparency and quality of domestic RWD collection, secure patient access and safety by generating reliable RWE, and establish an evidence-based expenditure management system. For example, post-listing control using RWE is expected to reduce uncertainties in clinical utility, cost-effectiveness, and financial impact of listed drugs, while strengthening patient access and safety with RWE-based post-listing control. It is also expected that the submission of standardized data prepared according to the guidelines will improve the predictability of pharmaceutical companies and shorten the review period for drug performance evaluation upon listing. The study will be conducted for 9 months from the date of selection and signing of the contract. The total budget for the study is KRW 100 million.
- Policy
- Daewon to sell AZ’s Symbicort in Korea
- by Lee, Tak-Sun Feb 06, 2025 05:56am
- Daewon Pharmaceutical will start selling AstraZeneca's asthma inhaler Symbicort (budesonide + formoterol fumarate hydrate) in Korea. The move is expected to expand the company's asthma inhaler lineup in addition to its existing Compona and expand the company’s market share. According to industry sources, Daewon Pharmaceutical will start selling Symbicort Turbuhaler -Symbicort Rapihaler this month. Symbicort, which was approved in Korea in 2001, has long been popular in the asthma inhaler market. Symbicort is a combination inhaler that combines the adrenocorticosteroid budesonide, which reduces inflammation, and formoterol, which dilates the airways in the lungs, and is used to treat asthma and severe COPD. The addition of Symbicort to Daewon's lineup is expected to increase its competitiveness in the domestic asthma inhaler market, which is worth about KRW 130 billion. Daewon launched Componacompact Air in 2020, a dry powder inhalation asthma treatment that combines fluticasone propionate and salmeterol xinafoate. Daewon Pharmaceutical had partnered with Turkish pharmaceutical company Neutec to import and sell the product, which is produced at Neutec's dedicated inhaler factory. The original fluticasone-salmeterol combination inhaler is GSK's Seretide. Together with Symbicort, the drugs held the market lead from 2000 until the 2020s. However, Seretide and Symbicort have since fallen from the top of the market due to the high effectiveness and ease of use of products such as Spiriva and Relvar. Last year, Seretide eventually withdrew from the Korean market. However, Symbicort's domestic sales are still over 10 billion won and its high market share is expected be beneficial for Daewon, which is a latecomer to asthma inhalers. In 2023, sales of Symbicort Turbuhaler had been KRW 7.9 billion and Symbicort Rapihaler KRW 6.7 billion, totaling at KRW 14.6 billion, according to IQVIA. “The asthma inhaler market had been a difficult one to enter for domestic pharmaceutical companies even after patent expiry as it requires inhaler technology,” said a pharmaceutical industry insider. ”Daewon entered the market with Compona in 2020 and is expected to expand its share by the addition of Symbicort.” Currently, there are only a few domestic pharmaceutical companies selling asthma inhalers, including Daewon Pharmaceutical, Kolon Pharmaceutical with Foster-Trimbow, Hanmi Pharmaceutical with Fluterol, and Huons with Zephirus. When narrowing down the products to domestically manufactured products, Fluterol is almost the only one.