- LOGIN
- MemberShip
- 2025-12-20 01:25:28
- Policy
- Temp reimb extension for antivirals including Tamiflu ends
- by Lee, Tak-Sun Jul 16, 2024 05:46am
- The limited reimbursement extension granted to antiviral drugs used for influenza such as Tamiflu has ended after 22 months. The program, which was introduced as a measure to prepare for the simultaneous spread of the COVID-19 and influenza pandemic, came to an end with the recent lifting of the flu pandemic warning. Now that the COVID-19 pandemic has subsided, the old reimbursement standard will be applied in the future in normal circumstances. According to the Health Insurance Review and Assessment Service (HIRA), the limited health insurance reimbursement coverage granted to influenza antiviral drugs, which had been in effect since September 13, 2022, ended on the 12th. The subject items were oral oseltamivir, such as Tamiflu Cap, and topical zanamivir, such as Relenza Rota Disk. Both drugs were used when the patient was confirmed positive for influenza through tests (rapid antigen test or polymerase chain reaction tests). However, when the influenza warning was issued, ▲patients under the age of 9, ▲pregnant or mothers within 2 weeks of giving birth, ▲65 years old or older, ▲immunocompromised, ▲metabolic disorders, ▲heart disease, ▲lung disease, ▲kidney dysfunction, ▲liver disease, ▲blood disorders, ▲neurological and neurodevelopmental disorders, and ▲patients under the age of 19 who are receiving long-term aspirin treatment were covered even without testing. In November 2021, the government temporarily extended health insurance coverage for antiviral drugs for suspected high-risk patients (pediatric, elderly, immunocompromised, etc.) even if they did not test positive and no influenza warning was issued, due to concerns about the outbreak of COVID-19 and influenza twindemic. The temporary extension was in effect from November 15, 2021, to June 20, 2022. Then, during the flu season, the measure was extended for a second time from Sept. 13, 2022, to July 12, 2024. The KDCA lifted the influenza pandemic warning for the 2023-2024 season on the 12th. As a result of the surveillance of influenza samples at the clinic level (300 centers), the number of suspected influenza patients fell below the epidemic threshold for 3 consecutive weeks, resulting in the decision to lift the flu pandemic warning after consultation with experts. Typically, the influenza season in Korea runs from November to April of the following year. If the number of cases remains below the epidemic threshold, it is unlikely that the reimbursement standard for antiviral drugs will be extended. In the case of Tamiflu Cap, the reimbursement extension and the flu epidemic resulted in KRW 15 billion in outpatient prescriptions (UBIST) last year. This was the highest in the last 5 years, and the explosive demand caused frontline pharmacies to struggle with supply. During the COVID-19 pandemic, on the contrary, sales were below KRW 5 billion as the flu waned. In 2020, sales recorded KRW 2.7 billion, and in 2021, no prescriptions were recorded at all. An industry official analyzed, "With the reduced flu epidemic and return to normal of the reimbursement standards, prescriptions for antiviral drugs such as Tamiflu will likely decrease.”
- Policy
- ‘Exclude GER and CAN or cut price by 50% less'
- by Lee, Tak-Sun Jul 15, 2024 05:48am
- As the government is pushing for external reference pricing reevaluations, the pharmaceutical industry has proposed two alternatives: excluding Germany and Canada from the A8 countries used for comparison or reducing the drug price cut rate by 50%. The government is reportedly reviewing the proposed options. The industry has taken a hardline stance, stating how it will not go through with the government’s external reference pricing reevaluation plan as is in its current state. According to industry sources on the 12th, the industry officials that participated in the 10th meeting for the external reference pricing reevaluations that was held on the 5th proposed the options above. The industry suggested that 6 countries (the U.S., Japan, the U.K., Switzerland, France, Italy, and the U.K.) should be used as reference, excluding Germany and Canada, which have different drug pricing systems from Korea and will inevitably render significant losses when comparing the countries’ public reimbursement benefits. Germany and Canada use external reference pricing systems. Under the reference pricing system, only generics below a certain price are granted listing, so the reimbursed public price of drugs is significantly lower than that of Korea, which may increase the losses rendered by domestic pharmaceutical companies. The pharmaceutical industry is also reported to have proposed a plan that reduces the drug price reduction amount by 50% when Germany and Canada are included. This is a method that has been applied in past external reference pricing reevaluations. "Including the public reimbursement prices applied in Germany and Canada in Korea’s reevaluation is quite unreasonable on the industry’s part," said an industry insider, adding, "Considering how the reductions in the drug price cut amount were applied in the past, it shouldn't be a big problem this time around.” The insider added, “The government is well aware of the damage that adding Germany and Canada will bring to the industry, so I believe it will accept one of the two options we have proposed today.” The current sources of external reference prices in the MFDS’s regulations are the U.S. Redbook (wholesale prices), the U.K. MIMS (pharmacy prices), the German Rote Liste (pharmacy prices), the French French Public Drug Database (ex-factory prices), the Italian Codifa (ex-factory prices), the Swiss Specialties List (ex-factory prices), the Japanese Ministry of Health, Labor and Welfare's Drug Price Standard (pharmacy price), and the Canadian PMPRB & Ontario Drug Benefit Formulary (ex-factory prices) However, the government's proposal became controversial because it had calculated the external reference price of drugs for reevaluation based on the price that is publicly reimbursed or similarly paid. Therefore, it is expected that the government will soon decide on the issue of applying the drug prices in Germany and Canada or reducing the cut amount by 50% and finalizing its draft.
- Policy
- Roche Korea speeds up reimb of Ocrevus in KOR
- by Lee, Tak-Sun Jul 12, 2024 05:48am
- Roche Korea is making active moves to receive reimbursement for its multiple sclerosis drug Ocrevus, which was approved in May this year. Roche, which applied for reimbursement immediately after the approval, has set out to persuade the Health Insurance Review and Assessment Service to expedite the application process. According to industry sources, Roche Korea recently applied for a drug presentation session to HIRA. The system was introduced in 2010 to increase the transparency and objectivity of drug evaluations by sharing information about new drugs between pharmaceutical companies and reviewers. The briefing is held between 1-2 months after the company applies for evaluations. However, for drugs that require supplemental data, a briefing is held after the supplemental data has been submitted and the reviewers have understood the submitted data. The briefing is open to reviewers and deputy directors involved in the evaluation of the reimbursement standard and the evaluation of the new drug. Roche requested to hold the briefing to provide reviewers with an accurate representation of the drug's efficacy and clinical utility and justify the drug’s reimbursement. Ocrevus is considered to have dramatically improved dosing convenience for patients with multiple sclerosis. As a recombinant humanized monoclonal antibody (mAb, IgG1) that selectively targets CD20-expressing B cells, it reduces the number and function of B cells, thereby inhibiting MS. MS is a chronic disease in which myelin sheaths are damaged by an autoimmune inflammatory response. Damage to the myelin sheath causes symptoms such as muscle weakness, fatigue, and vision impairment, and can lead to non-traumatic neurological disability. An estimated 1,800 patients are known to be living with MS in Korea. Previously, patients had to receive weekly injections such as beta interferon to relieve their symptoms. Ocrevus, on the other hand, is said to be more convenient to administer as the first dose of 600 mg is divided into 2 intravenous infusions, and the 600 mg doses thereafter are administered as a single intravenous infusion every 6 months. The drug was approved by the U.S. FDA in 2017 and its subcutaneous formulation recently received EU approval. A barrier to its reimbursement in Korea is the high price of the drug. In the U.S., the annual cost of the drug is nearly KRW 100 million. Although it is expensive, the company likely applied for the briefing to emphasize the justification for its reimbursement. In particular, there are observations that Roche may be rushing to receive reimbursement as Celltrion is avidly developing a biosimilar of the drug. An industry insider explained, "Through the drug briefing, pharmaceutical companies can appeal to not only the reviewers but also the relevant deputy directors on how just the reimbursement is, and share complementary ideas on key issues, which can speed up the submission of documents in the future reimbursement review process."
- Policy
- Reassessing the plan for comparing foreign drug prices
- by Lee, Tak-Sun Jul 12, 2024 05:48am
- The government will likely reassess foreign drug price comparison by evaluating the therapeutic category with the highest number of products. Consequently, gastrointestinal agents, high blood pressure drugs, and antibiotics will be assessed in the first year. There are over 2000 products in these three therapeutic categories. Furthermore, drugs with the same ingredient products produced by fewer than three companies will likely to be excluded from the assessment. According to industry sources on July 10th, the government is gathering opinions from pharmaceutical companies about the current reassessment plan, which has been shared through ten meetings with the pharmaceutical industry (as of July 5th). The current reassessment plan is not the final one. The issue is still ongoing because it does not include the referencing sources of Germany and Canada. In the first year, according to the reassessment plan, therapeutic categories with the highest number of products, including gastrointestinal agents (2043 products), high blood pressure drugs (2268 products), and antibiotics (2156 products), will be assessed. In the second year, hyperlipidemia drugs, respiratory system medications, central nervous system medications, diabetes drugs, and musculoskeletal disorder medications will be assessed. In the third year, ophthalmology drugs, otolaryngology drugs, dentistry drugs, painkillers, urological and reproductive system medications, anticoagulants, dermatological treatments, anticancer agents, and 17 other therapeutic categories will be assessed. The current plan to reassess foreign drug price comparison (as of July 5th). (Therapeutic category with the highest number of products) In the first year, gastrointestinal agents (2043 products), high blood pressure drugs (2268 products), and antibiotics (2156 products), will be assessed. In the second year, hyperlipidemia drugs, respiratory system medications, central nervous system medications, diabetes drugs, and musculoskeletal disorder medications will be assessed. In the third year, ophthalmology drugs, otolaryngology drugs, dentistry drugs, painkillers, urological and reproductive system medications, anticoagulants, dermatological treatments, anticancer agents, and 17 other therapeutic categories will be assessed. However, exclusion will be applied to ▲low-priced drugs, orphan drugs, drugs that have been listed as shortage prevention drugs (SPD) ▲Oxygen, nitrogen dioxide, saline solutions, artificial perfusion agents, and radioactive drugs ▲Narcotics ▲Drugs with the same administration, ingredients, and formulation produced by fewer than three companies ▲Products undergone price increases (after January 2020). During the discussion, drugs with the same administration, ingredients, and formulation produced by fewer than three companies were added to the list. A reference price for adjustment is the average price of drugs found in A8 countries. Adjusted mean price will exclude the highest and the lowest prices. Products with a higher drug price than the calculated reference amount will be subjected to a reduction. In cases where fewer than 2 out of 8 countries can be found, the average reduction rate of the most similar product is applied, considering factors such as content, ingredients, formulations, and administration methods. Products with generic prices higher than the standard amount calculated using the highest price within the same product will be reduced. When calculating the average reduction rate, if a negative reduction rate occurs due to lower prices compared to foreign countries, it will be reflected as such in the calculation. For combination therapies, if the assessed amount is lower than the sum of the assessed amounts for monotherapy or combination therapy, the reduction will be limited to the sum of the amounts assessed. The prices of pharmaceuticals that have submitted the required documents will be reduced to the amount assessed for their intended development products. Furthermore, it has been revealed that the current plan included the addition of criteria stating that the calculation of price adjustments referencing foreign drug prices should be based on public prices reimbursed or reimbursed equivalently in the respective country. The pharmaceutical industry's significant opposition to the public reimbursement requirements during the tenth meeting may prompt revisions in the final plan.
- Policy
- ‘Bioequivalence tests rather intensified price cuts'
- by Lee, Tak-Sun Jul 12, 2024 05:47am
- The pharmaceutical industry is calling for improvements as their drugs that have completed bioequivalence tests per the government’s insurance price ceiling reevaluations may face larger price cuts under ‘Type C’ of the Price Volume Agreement negotiations this year. Generic drugs that have completed bioequivalence tests during the insurance price ceiling reevaluations have maintained their existing prices. On the other hand, the price of a number of drugs that were unable to demonstrate bioequivalence was cut, leveling the weighted average price of the same ingredient drugs (substitutes) downward. The higher the price of the drug in negotiations as compared to the weighted average price of its substitutes, the greater the price reduction, putting the bioequivalent drugs at a relative negotiating disadvantage. According to industry sources on the 11th, the price difference between the weighted average price of the negotiated drug and the weighted average price of the equivalent drugs became larger and is expected to increase the price reduction of the respective drugs during PVA negotiations. According to the government’s Detailed Operating Guidelines for PVA Negotiations, the weighted average price of substitute drugs is one of the considerations used to adjust the reference price used in negotiations. According to Article 11 of the guidelines, considerations for adjusting the negotiated reference price include: ▲ the impact of the increase in claims of the negotiated drug has on the reduction or increase in insurance finances ▲ the impact of the negotiated drug on the weighted average price of the entire market, which includes substitute drugs. The substitute drugs for ‘Type C’ PVA are drugs with the same ingredient and route of administration as the negotiated drug. As the weighted average price of same-ingredient drugs has been leveled downward due to price ceiling reevaluations, their impact on the weighted average price of negotiated drugs whose prices have been maintained by demonstrating biological equivalence is bound to increase. "As the weighted average price of substitute drugs has been leveled downward, the bioequivalent drugs may be regarded to have an adverse effect on national health insruance finances, resulting in been larger price cuts than that made using the reference formula during PVA negotiations.” said a pharmaceutical industry representative. In other words, the greater the price difference is between the negotiated drug and the weighted average price of its substitutes, the greater the price reduction may become with increased usage. The generic drugs that have demonstrated bioequivalence will be losing out because their price was maintained during the previous negotiations, rendering the price difference greater compared with the weighted average price of its substitutes, which were leveled downward. The pharmaceutical industry has been expressing frustration as the bioequivalence tests they conducted to comply with government policy are being used against them. The insurance price ceiling reevaluations on generics took place last year and this year to improve the quality of generic drugs. The government pulled out the bioequivalence card to improve quality after a number of generic products were found to contain NDMA and other carcinogens in their ingredients, including the hypertension drug valsartan. Generics that have proven their bioequivalence were allowed to maintain their prices, while generics that were easily approved through contract manufacture had their prices reduced. The government’s intention at the time was to save only those drugs that took the time and expense to prove their viability from penalties, but instead, the bioequivalent drugs are being penalized through the post-marketing control system. In response, the industry is calling on the government to find a way to improve the system so that those who cooperated with government policy are not disadvantaged during PVA negotiations. For example, the government plans to negotiate PVA drugs to reflect the contribution of items that increased production or were used to treat infectious diseases through information cooperation to address the unstable drug supply and demand issue last year. The pharmaceutical industry believes that the PVA guidelines should also reflect the drugs’ contribution to improving drug quality. "The more the items that have not demonstrated bioequivalence there are, the lower the weighted average price of substitute drugs, and the more expensive the bioequivalent drugs may appear to be, resulting in a larger negotiated price cut," said an industry official. "It is unreasonable to try to further cut drug prices just because they are relatively expensive during PVA negotiations, even though they are being well sold for abiding with government policy."
- Policy
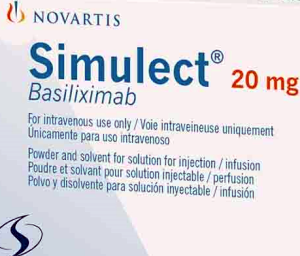
- Simulect in supply crisis with no alternative
- by Lee, Tak-Sun Jul 11, 2024 05:47am
- Simulect Inj (basiliximab, Novartis Korea), which is used as an induction therapy to prevent immune rejection in organ transplant patients, is expected to run out of supply in Korea due to issues in securing the supply the drug’s active pharmaceutical ingredient. In particular, there is no alternative drug for liver transplantation, increasing the need for the government’s prompt response. Novartis Korea reported to the Ministry of Food and Drug Safety on the 8th that the company expects a supply shortage due to the lack of global supply of the API for Simulect Inj. Due to the API issue, the amount scheduled to be imported in September will not arrive. As a result, the company expects the remaining stock to run out by mid-January next year based on the current inventory. Simulect is a leading induction therapy drug used to prevent immune rejection during organ transplantation. It is reimbursed for kidney, heart, liver, lung, small bowel, pancreas, and pancreatic islet transplants. The company noted that while hospitals and transplant centers may be able to reschedule living donor transplants, which account for approximately 70% of all transplants, in the short term, a shortage of Simulect could result in changes in the physical condition of the donor and recipient during the delay, which could affect the outcome of the transplant and result in the postponement of all other scheduled surgeries. The company added in the case of the rest - the 30% deceased donor transplants - timing is critical due to the nature of the donor, so the surgery must be performed quickly, and as surgery is nearly impossible without induction therapy, both the organ donation and the surgery may be rendered impossible. The problem is liver transplants. There are currently no other approved or available drugs for induction therapy for liver transplants, although around 1,500 cases are performed per year. In the worst-case scenario, liver transplants could become unavailable. Novartis plans to accelerate the approval of its new finished product manufacturing site. By the end of August, it could begin shipping products manufactured in Italy. However, domestic approval has not been granted for imports from that plant yet. "As the new manufacturing site can be put into producing finished products upon approval of the plant change request, we will work to minimize the supply shortage period through various measures, including efforts to accelerate the approval of the request.” In March, Simulect was also hit with a three-month import suspension when it recalled products due to possible glass particle contamination in the attached ampoules. However, the company responded quickly and did not disrupt supply. However, as this is a global API supply issue, if the administrative process of replacing the manufacturing plant is delayed, domestic supply and demand instability is deemed inevitable. This is why appropriate support from regulatory agencies such as the Ministry of Food and Drug Safety is necessary.
- Policy
- Debate within the Health and Welfare Committee members
- by Lee, Jeong-Hwan Jul 10, 2024 05:48am
- Members of the Democratic Party of Korea and the ruling party who are also committee members of the Health and Welfare Committee of the National Assembly show significant differences in viewpoints regarding President Yoon Suk Yeol’s policy of expanding medical school quotas, and the current situation surrounding the conflict between the medical community and the government, and the medical crisis. The ruling party argues that the National Assembly must support the government’s medical reforms to increase the number of doctors and help essential and regional medical services. The Democratic Party of Korea argues that the government must first clearly take responsibility for the medical exodus caused by forced expansion. As the viewpoints of the ruling and opposition parties diverge on government's medical reforms, such as increasing the medical school quota, it has become increasingly difficult to establish a bipartisan deliberation committee to resolve conflicts between the medical community and the government. On July 9th, members of the People Power Party and the Democratic Party, who are committee members of the Health and Welfare Committee, held a separate press conference and criticized the other party. The Democratic Party says, "The government must admit to causing the medical community-gvt conflicts and medical crisis" During the press conference, the Democratic Party condemned the People Power Party's withdrawal from adopting a bipartisan resolution and opposing the specification of 'government responsibility during the emergency hearing on the medical crisis. They argue that the People Power Party is responsible for the 13-hour hearing without a result or resolution. The Democratic Party argued that the Presidential Special Committee on Medical Reforms is clearly limited in capacity and appealed for the need to establish a public discussion committee on medical reforms involving both parties, the government and experts. Kang Sunwoo, Secretary of the Democratic Party, said, "We are disappointed in the ruling party's refusal to take even minimal responsibility to correct the government's clear policy failures." He emphasized, "The People Power Party held a superficial hearing in Yongsan, not for the people." The People Power Party says, "Medical reforms, both parties must collaborate instead of political conflicts" In the afternoon of the same day, the People Power Party held a press conference to refute the Democratic Party's claims. The party asserted that the Democratic Party is politicizing medical reforms to improve essential healthcare and regional healthcare into a political issue. The People Power Party said that previously, the Democratic Party government had pursued increasing medical school admissions but was hindered by opposition from the medical community. They emphasized that achieving medical reforms, a challenging task, requires collaborative efforts across government administrations, regardless of political affiliation. They argued that the Democratic Party must stop arguing if it hopes to resolve the medical crisis quickly and improve essential and regional healthcare. Kim Miae, Secretary of the People Power Party, said, "Instead of addressing patient anxiety caused by trainee doctors leaving and medical staff going on strike, the Democratic Party, during a press conference, claimed that the ruling party unilaterally rejected the resolution." She added, "The Democratic Party is trying to politicize the current situation and emphasize only government responsibility as a tactic. We must put joint efforts into problem-solving rather than politicizing the issue." Meanwhile, the Health and Welfare Committee will commence a general meeting on the upcoming 16th and listen to work reports from the government departments, including the Ministry of Health and Welfare (MOHW) and Ministry of Food and Drug Safety (MFDS). During the meeting, the Democratic Party of Korea and the ruling party are expected to participate in questioning and discussion to formulate a solution to resolve the conflict between the medical community and the government and the medical crisis.
- Policy
- Reimbursement application submitted for 'Adempas tab'
- by Lee, Tak-Sun Jul 10, 2024 05:48am
- Bayer’s Bayer’s 'Adempas tab,' a treatment for chronic thromboembolic pulmonary hypertension (CTEPH), is being considered for reimbursement listing 10 years after its approval as a novel drug. According to industry sources on July 2nd, a reimbursement decision application for Adempas was recently submitted to the Health Insurance Review and Assessment Service (HIRA). This drug obtained approval in South Korea as an orphan drug in June 2014. Five products with different doses are available, and it has the efficacy and the effect in ▲patients with persistent/recurrent chronic-thromboembolic pulmonary hypertension (CTEPH, WHO Group 4) after surgical treatment or inoperable CTEPH, to improve exercise capacity ▲adult patients with arterial pulmonary hypertension (PAH, WHO Group 1) who have WHO functional class 2-3, to improve exercise capacity. Adempas has been known as the first novel drug to treat CTEPH. CTEPH occurs in patients with chronic pulmonary embolism who progress to chronic obstructive pulmonary disease (COPD) and develop fibrotic stenosis and occlusion, leading to pathological vascular remodeling and increased resistance in the pulmonary arteries. CTEPH is a chronic disease that causes progressive shortness of breath and right heart failure. Symptoms include dyspnea, fatigue, chest pain, dizziness, peripheral edema, cough, and hemoptysis, significantly impacting quality of life. Ultimately, it can progress to heart, kidney, and liver failure, potentially leading to death. Treatment options include pulmonary endarterectomy (PEA) surgery. However, 40% of patients are ineligible for surgery, making drug therapy crucial. Therefore, Adempas was highly anticipated to be the first drug. It has been 10 years since it was approved in South Korea. However, patients had high economic burden because the drug was not covered by the National Health Insurance reimbursement. The industry attributes the drug’s case to the low drug price. According to IQVIA, the drug’s sales performances for the past five years remain at zero. However, Bayer applied for reimbursement 10 years after obtaining the approval. "In clinical trials, Adempas improved symptoms and occurrence rate in CTEPH patients, and it also demonstrated an effect in alleviating shortness of breath," a Bayer representative said. "When the drug becomes reimbursement listed, it will significantly help patients."
- Policy
- 1st bispecific antibody Epkinly approved with a condition
- by Lee, Hye-Kyung Jul 09, 2024 05:51am
- 'Epkinly (epcoritamab),' the first T-cell–engaging bispecific antibody, was found to have been approved under the condition that the company submits Phase III trial data. In Korea, the approval was granted on March 20 based on Phase II clinical trial data and a Phase III trial protocol, but at the time, a condition was added that the company must submit data from a therapeutic confirmatory trial. According to the minutes of the Central Pharmaceutical Affairs Committee meeting held in April, which were recently released by the Ministry of Food and Drug Safety, Epkinly was granted conditional approval and was required to submit Phase III trial results after the approval of the product by applying Article 24, Paragraph 3 of the 'Regulations for Approval and Review of Biological Products, etc. 'Epkinly (epcoritamab),’ which is imported by Abbvie Korea, is a bispecific monoclonal antibody that binds to both the CD3 on the surface of T-cells and CD20 on the surface of B-cells and is indicated for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) after 2 or more lines of systemic therapy In Korea, Polivy, Kymriah, Selinexor, and Columvi are approved for the treatment of patients with relapsed or refractory DLBCL. DLBCL is one of the most common blood cancers and is one of the most common types of widely spread (diffuse) non-Hodgkin lymphoma, characterized by rapid progression. Epkinly binds to CD3 to activate T cells and binds to CD20 to bring B cells to the side of activated T cells and induce B cell death. In this regard, a CPAC member said, "In a clinical trial in patients with persistent relapses requiring 3 or more cycles of treatment, Epkinly demonstrated some degree of effectiveness and safety. Its effectiveness and safety have been confirmed over other existing drugs, so we deemed granting conditional marketing authorization was appropriate pending the results of its Phase III clinical trial." When the U.S. FDA granted the drug through its accelerated approval program, the approval was based on the Phase I/II EPCORE NHL-1 study. In 148 patients with relapsed or refractory DLBCL who had received a median of 3 prior therapies, the drug demonstrated an objective response rate of 61%, a complete response rate of 38%, and a median duration of response of 15.6 months in patients with CD20-positive diffuse large B-cell lymphoma. Another CPAC member added, "Based on the Phase II results, Epkinly appears to be an effective and safe agent when compared to other 2nd or later line agents. Therefore, we believed the conditional approval was warranted, subject to submission of its Phase III results."
- Policy
- BMS’ Camzyos and Handok’s Empaveli pass the DREC review
- by Lee, Tak-Sun Jul 09, 2024 05:51am
- Bristol Myers Squibb Korea’s 'Camzyos Cap,' a treatment for symptomatic obstructive hypertrophic cardiomyopathy (oHCM), has passed the Drug Reimbursement Evaluation Committee (DREC) review after reconsideration. Handok’s 'Empaveli Inj,' a treatment for paroxysmal nocturnal hemoglobinuria (PNH), also obtained approval for reimbursement and moved on to negotiations. The Health Insurance Review and Assessment Service (HIRA) commenced the 7th Drug Reimbursement Evaluation Committee (DREC) of 2024 and announced that it had decided the appropriateness of reimbursement for these drugs. The review outcome of assessing the appropriateness of benefit coverage for drugs that have applied for a decision: Three pharmaceuticals have been considered for this round of DREC review. The symptomatic oHCM treatment, Camzyos Cap, a PNH treatment, Empaveli Inj, and an NMOSD treatment, Uplizna Inj, received the assessment. Among these, Camzyos Cap and Empaveli Inj obtained appropriateness of reimbursement decisions. Two drugs are expected to undergo drug pricing negotiations with the HIRA and will receive the final decision for reimbursement listings. Camzyos received the reconsideration decision from the DREC review held last month. In just one month, DREC granted the reimbursement appropriateness. On the other hand, Uplizna Inj received a decision that it will meet the appropriateness of reimbursement if the company accepts an amount below the standard. If Mitsubishi Tanabe Pharma accepts an amount below the standard, the drug will be considered for negotiations with the HIRA. If the company fails to take it, the drug will not be listed for reimbursement. If the company accepts an amount below the standard for exemption from the drug pricing negotiations, it will only negotiate the expected claim amount.