- LOGIN
- MemberShip
- 2025-12-20 21:52:06
- Policy
- Samsung Bioepis’s Soliris biosimilar is introduced
- by Lee, Tak-Sun Mar 25, 2024 05:59am
- The cost of Soliris (eculizumab), a drug known for its high price, is expected to drop significantly with the introduction of its biosimilar. The original Soliris will also cut its price by 30% in line with the expansion of its reimbursement benefit to cover neuromyelitis optica, to compete with its biosimilars. According to industry sources on the 22nd, Samsung Bioepis' Soliris biosimilar ‘Episcli Inj’ will be reimbursed from April. The upper limit will be set at KRW 2,514,858 per vial. This is only 48.8% of the current upper limit set for Soliris, which is set at KRW 5,132,364. The 1-year drug cost of Episcli Inj is also KRW 271 million, half the cost of Soliris' KRW 554 million. As a result, the burden on patients is expected to be significantly reduced if the patients choose to use biosimilars. However, Soliris is also taking measures to gain a competitive edge. The drug’s reimbursement was extended to cover neuromyelitis optica spectrum disorder (NMOSD). Currently, it covers orthopedic hemolytic uremic syndrome (aHUS) and paroxysmal nocturnal hemoglobinuria (PNH). During negotiations with the National Health Insurance Service to expand the scope of coverage, Soliris’s company agreed to reduce the upper limit by 29.9%. Starting next month, the upper price limit of the drug will be reduced to KRW 3.6 million per vial. The cost of a year's supply will also be reduced from KRW 554 million to KRW 288 million. Patients will pay 10.5 million won per person per year when applying the copayment ceiling. Soliris' decision to lower the drug price was also influenced by the reimbursed entry of Enspryng (satralizumab), its competitor in optic neuromyelitis. Enspryng has been reimbursed as of December 1 of last year. Its out-of-pocket cost per patient per year is up to KRW 101.4 million when applying the copayment ceiling system. If Soliris’s price is reduced, its out-of-pocket cost will no longer be significantly different from Enspryng.
- Policy
- Economic benefits paid to doctors to be publicly disclosed
- by Lee, Jeong-Hwan Mar 22, 2024 06:08am
- On the 21st, the government announced the guidelines for the public disclosure of expenditure reports on the legitimate financial benefits paid by pharmaceutical and medical device companies to doctors and pharmacists that will be disclosed in December. The guidelines stipulate the detailed schedule, content, and method for disclosure of the economic benefit expenditure report prepared by companies as of the fiscal year 2023, per the amendments to the Pharmaceutical Affairs Act and the Medical Device Act, made on July 20, 2021. Under the current law, the legally permissible economic benefits that can be provided are the provision of physical products, support for conferences, support for clinical trials, product presentations, post-marketing surveys, cost discounts based on payment terms, and use for performance verification before purchase (medical devices only). When the expenditure report is disclosed, the public will be able to check the medical institution (name, symbol of the medical institution) that received economic benefits from drug and medical device suppliers, information on support for conferences, and the amount of support provided to participants at product presentations. However, under the Enforcement Rules of the Pharmaceutical Affairs Act and the Medical Device Act, the names of recipients such as medical practitioners whose personal information is at risk of exposure and clinical trial information containing confidential business strategies will be de-identified and disclosed in the expenditure report. This is in accordance with Article 44 (2) (3) of the Enforcement Rule of the Pharmaceutical Affairs Act, which stipulates, "If the Minister of Health and Welfare is notified that the details of the expenditure report contain information subject to non-disclosure under Article 9 of the Official Information Disclosure Act, it shall be disclosed after taking necessary measures to ensure that the contents cannot be identified. Kyung-sil Chung, Director Bureau of Healthcare Policy at the Ministry of Health and Welfare (MOHW), said, "The disclosure of the expenditure reports is to create an environment for the industry to self-censor rebates through the disclosure of legitimate economic benefits. We will be receiving related data through the Health Insurance Review and Assessment Service from June this year, and we ask for the companies’ cooperation to ensure accurate information disclosure." The operating guidelines for the expenditure report will be available on the websites of HIRA and other related associations.
- Policy
- Enhertu’s price falls to KRW 70 mil range with reimb
- by Lee, Tak-Sun Mar 22, 2024 06:06am
- The list price of the petitioned anti-cancer drug Enhertu was reportedly set at KRW 1.4 million per vial. This is significantly cheaper than the non-reimbursed price of KRW 2.3 million. In addition, the National Health Insurance Service (NHIS) will apply a risk-sharing agreement (RSA) system for the reimbursement of the drug, so the actual price of the drug is expected to be lower. According to industry sources on the 21st, Enhertu’s price is going to be set at an upper limit of about KRW 1.4 million per vial. The price had been reportedly applied to the drug when it passed HIRA’s Drug Reimbursement Evaluation Committee review in February. In other words, the NHIS seems to have been negotiating around the price set by DREC. This may explain why the negotiations began in late February and were completed in just a month. Considering that an average weight patient requires 3 vials per treatment cycle (21 days), the drug’s non-reimbursed price is about KRW 7 million, and the listed reimbursed price is KRW 4.2 million. In terms of annual treatment costs, the non-reimbursed price is about KRW 120 million and the list price is about KRW 71.4 million. The actual price is likely to be lower with the application of the RSA system. The expenditure cap type and refund type will be applied to Enhertu. Enhertu’s reimbursement listing will be officially notified after being reported to the Health Insurance Policy Deliberation Committee's meeting scheduled for next week. Enhertu is an antibody-drug conjugate (ADC) anticancer drug that is highly effective against HER2-positive breast cancer, which accounts for around half of all metastatic breast cancers. It is also used for HER2-positive gastric or gastroesophageal junction (GEJ) adenocarcinoma. In particular, the drug demonstrated a better effect over existing drugs in clinical trials. Compared to chemotherapy, Enhertu reduced the risk of disease progression or death by 50%. The overall survival (OS) was 23.4 months with Enhertu, 6.6 months longer than the 16.8 months of the control group. The superior effect prompted the call for fast-track registration of Enhertu in the medical field. The petition was signed by 50,000 people and reported to the National Assembly.
- Policy
- National Essential Medicines to be categorized into 4 groups
- by Lee, Hye-Kyung Mar 21, 2024 05:53am
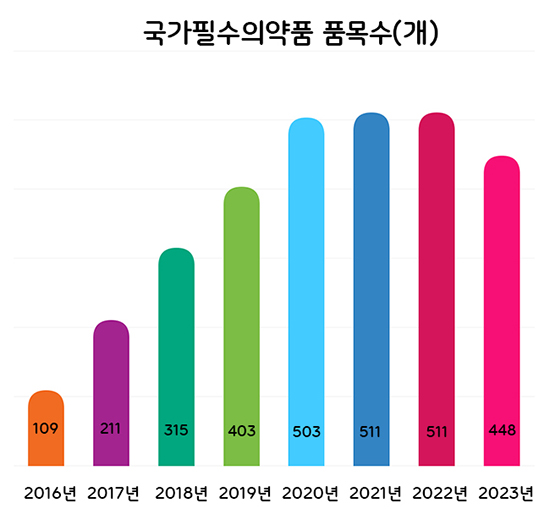
- The Ministry of Food and Drug Safety (MFDS) plans to categorize national essential drugs into 4 groups and conduct regular level assessments to better manage the drugs. According to the 'Research Service to Prepare a Measure to Classify and Manage the National Essential Medicine ‘ that was recently announced by MFDS, the authorities will discuss improvements and revisions to the classification criteria used to set the supply stability grade of national essential drugs by May. The four-level classification plan was made in response to the need to establish a differentiated management system for already designated national essential drugs, which was pointed out in the 2nd Comprehensive Plan for Stable Supply of National Essential Drugs in 2022. Through the project last year, the MFDS prepared four-level classification criteria for national essential drugs: ▲drugs that require prior stockpiling, ▲drugs that require immediate supply in case of a crisis, ▲ drugs that require constant monitoring of the supply situation and promotion of localization, and ▲drugs that require continuous attention. The classifications are based on the impact on public health, urgency of use, and potential availability of supply. Number of National Essential Medicines by Year Based on the research service results, the MFDS will finalize the classification and management methods of already designated national essential medicines and prepare feasibility studies and designations for ingredients and formulations that request new designations. For already designated national essential drugs, the MFDS plans to apply the 4-level classification system and conduct regular level management. For this, the research will identify and revise improvements to the classification system. The proposed list of ingredients and formulations that are applied to the standards and improvements prepared through the research will be reviewed by the National Essential Medicine Subcommittee. Ingredients and formulations requesting new designations this year will be reviewed for healthcare necessity and supply stability based on the evaluation criteria that had been determined through the Council for the Safe Supply of National Essential Medicines that was in November last year. The MFDS explained, "We need to continue to designate drugs that are essential for healthcare but are difficult to be supplied stably through market functions alone as national essential medicines and update the list of already designated national essential drugs. The final ingredients and formulations will be designated after resolution by the Council for the Safe Supply of National Essential Medicines Meanwhile, the number of national essential medicines has steadily increased from 109 in 2016 to 511 in 2022, and a total of 448 ingredients and formulations have been designated through new and de-designations last year.
- Policy
- Gov’t raises price of Harmonilan due to unstable supply
- by Lee, Tak-Sun Mar 21, 2024 05:52am
- Enteral nutrition products Harmonilan(left) and Encover(right) The insurance ceiling price of the enteral nutrition formula Harmonilan (B Braun Korea), which the company has been experiencing difficulties in ensuring supply and demand recently, is expected to rise from April. The company had continuously been applying to register the drug as a ‘drug shortage prevention drug' and preserve production costs due to its low profitability, to no avail. This is why criticism arose on how the government has deferred taking measures until now and only set out to adjust the price ceiling after the supply and demand instability intensified. Harmonilan’s actual transaction price had been cut 2 years ago According to industry sources on the 20th, the company has completed negotiations to raise the ceiling price of Harmonilan with the government, and the new drug price is expected to be applied next month after being reported to the Health Insurance Policy Deliberation Committee. Encover and Harmonilan are the two products that dominate the enteral nutrition market. Enteral nutrition products are given to patients who have difficulty getting nourished through food. The product is directly into the digestive tract through a tube. They are essential for patients with eating disorders. However, enteral nutrients such as Harmonilan and Encover are often in short supply depending on import situations, which has caused a lot of frustration among patients. This is why healthcare providers and patients have long requested such enteral nutrition products be designated as a drug shortage prevention drug, but health authorities have been unwilling to do so. Pharmaceutical companies have also requested the designation. Because they are low-margin products, the companies explained that they would need to raise the price of the drugs to conserve their cost and keep up with the high demand. However, the price of these enteral nutrition products has fallen in the past due to actual transaction price cuts and the Price-Volume Agreement system. The price of Harmonilan 200ml was lowered from KRW 2,291 to KRW 2,282 in January 2022 as part of actual transaction price cuts. In the case of Encover, the price of the 200ml product had fallen 2.9% in October 2022 under the PVA system. The company had applied for price adjustments at the time, but the price had not been increased then. Such price reductions are regarded as a major cause of supply instability, as they decrease profitability. Therefore, the price increase granted for Harmonilan this time is regarded as one example of the government's inconsistent drug price adjustment policy. Drug that require price increases are also subject to price cuts The government has been postponing the implementation of the actual transaction price cut plan, which had been scheduled to take effect in January. This in part is interpreted as the government’s effort to to prevent cases like Harmonilan, as drugs with unstable supply and demand may be subject to price reductions. According to government officials, it is likely that the price reduction will not be implemented in April either. The postponement has also halved the health insurance financial savings the government sought to earn through the drug price reduction. The government seems to be in a dilemma, caught between its recent move to broadly raise drug prices of drugs with unstable supply and measures previously prepared to cut the prices of existing drugs. The industry is calling for a complementary measure to prevent price cuts not only for existing drug shortage prevention drugs but also for drugs that are likely to have unstable supply and have difficulty continuously treating patients. An industry official said, "The list of non-eligible items should be expanded after close examination when selecting subjects for actual transaction price cuts and PVA price cuts reductions. We need to get rid of the absurd situation where a drug subject to a price reduction suddenly becomes a drug subject to a price increase.”
- Policy
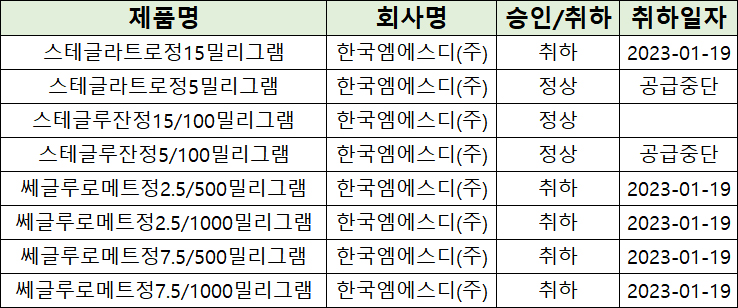
- MSD withdraws SGLT2 inhibitors in a row
- by Lee, Hye-Kyung Mar 20, 2024 05:44am
- MSD Korea is facing tough competition in the market for SGLT-2 inhibitors in South Korea. MSD decided that they will discontinue the supply of Steglatro Tab 5 mg and 'Stegluzan Tab 5/100 mg (ertugliflozin plus sitagliptin)' after voluntarily withdrawing 'Steglatro Tab 15 mg (ertugliflozin)' and metformin combination therapy, 'Segluromet,' last year. The list of approved and withdrawn SGLT-2 inhibitors by MSD in South Korea. Except for Stegluzan Tab 15/100 mg, all of MSD According to the Ministry of Food and Drug Safety (MFDS) report on the 15th, supply discontinuation was reported for Steglatro Tab 5 mg and Stegluzan Tab 5/100 mg. Imports of Steglatro Tab 5 mg will end on May 31st and Stegluzan Tab 5/100 mg will end on August 30th, after which these drugs will be discontinued. MSD says, “Due to a reduction in market demand, we will discontinue these drugs after the final import.” And MSD also stated, “The last import date is flexible depending on the manufacturing schedule and planned between mid-May and mid-August.” MSD stated that substitution is possible since other SGLT-class products are already available in the market. They also mentioned that they will notify healthcare professionals before discontinuing sales to ensure appropriate measures can be taken. If MSD voluntarily withdraws two items that they have decided to discontinue importing, Stegluzan Tab 15/100 mg will be their only SGLT-2 inhibitor containing ertugliflozin. However, Stegluzan Tab 15/100 mg has no distribution record after approval, and the company does not intend to distribute. The market for SGLT-2 inhibitors has grown to KRW 170 billion. According to the pharmaceutical market research agency UBIST, Steglatro generated merely KRW 1.6 billion in outpatient prescriptions. Industry analysis suggests that Steglatro lacks a competitive edge compared to Forxiga and Jardiance because its indication is limited to type 2 diabetes and clinical trials are lacking in expanding treatment areas. Changes in prescription performance by SGLT-2 inhibitors. SGLT-2 inhibitors have emerged as treatments for type 2 diabetes and are broadening their use in various diseases targeting the heart and kidney. SGLT-2 inhibitors inhibit the reabsorption of glucose in the kidneys, leading to glucose excretion in the urine. This results in reduced blood sugar, weight loss, kidney function protection, and blood pressure reduction. As of 2022, the items have recorded over KRW 40 billion in outpatient prescriptions individually, including AstraZeneca’s monotherapy 'Forxiga (ingredient: dapagliflozin),' a combination therapy ‘Xigduo,’ and Boehringer Ingelheim's monotherapy Jardiance (ingredient: empagliflozin). AstraZeneca’s drug generated KRW 91.4 billion, approximately KRW 15 billion more than Boehringer Ingelheim’s KRW 76.1 billion.
- Policy
- President Yoon stands on expanding quota to 2,000
- by Lee, Jeong-Hwan Mar 20, 2024 05:44am
- President Yoon Suk Yeol. President Yoon Suk Yeol reaffirmed the government’s plan to increase the medical school enrollment quota to 2,000 and stated, “Korea’s policy related to the number of doctors is not meeting the standards of the current era and actual needs, repeating the history of failure. Doctors’ licenses should not be used as a tool to intimidate Korean citizens and raise anxiety.” President Yoon presided over a Cabinet meeting at the presidential office in Yongsan and stressed, “The medical reform we are facing is our duty for the benefit of citizens, and it is a request from citizens.” And adding, “Korea’s policy related to the number of doctors is not meeting the standards of the current era and actual needs, repeating the history of failure.” “Delaying the expansion will eventually result in greater harm to the Korean citizens,” Yoon said. “Moreover, a much larger expansion will be needed in the future, and social controversies and conflicts surrounding medical reforms will also intensify every year. If citizens must constantly plead with their doctors for healthcare, can we truly say that the country is functioning properly?” In particular, “A stepwise approach or delaying expansion will not save the lives of the citizens nor accomplish medical reform to stop the collapse of regional and essential healthcare,” Yoon explained. “I hope trainee doctors and doctor organizations participate in a discussion about detailed measures to implement medical reform instead of engaging in fights outside of hospitals,” Yoon said. “I have been leading government-public discussions and will continue to hold sessions about medical reform in the form of government-public discussions.” Finally, Yoon mentioned visiting Asan Medical Center in Seoul to meet with hospital officials, pediatric obstetricians, and nurses. “I hope the medical staff who left will return to patients and join these staff,” Yoon said. “I will make a promise as a president to talk face-to-face with citizens about medical reform.”
- Policy
- Essential medical devices will be designated by next year
- by Lee, Hye-Kyung Mar 20, 2024 05:44am
- The Ministry of Food and Drug Safety is considering establishing a system to designate national essential medical devices. More specifically, the government plans to set a specific scope of medical devices that are essential for healthcare, such as rapid antigen test kits and life support devices that were necessary during COVID-19 but were difficult to supply stably through the market’s function alone. Nam-hee Lee, Director-General of Medical Device Safety at MFDS At a briefing with the MFDS’s correspondent journalists briefing, Nam Hee Lee, Director-General of the Pharmaceutical Safety Bureau at MFDS, said, "The need to designate essential medical devices has emerged as with pharmaceuticals amid the COVID-19 crisis. However, no specific scope has been set for the medical devices, and it is necessary to discuss how to designate local or existing licensed medical devices, so we will need to conduct a study first." For pharmaceuticals, the Pharmaceutical Affairs Act defines national essential medicines, and the government has designated and is managing 448 items as national essential medicines as of 2024. "There are frameworks set under the law to manage rare medical devices and medical devices subject to supply interruption reports, but the COVID-19 crisis has revealed global supply chain issues and that we rely on overseas import for many medical devices. In part, we need to localize medical devices, and there are medical devices we have the technology for but are not produced due to profitably issues, so we need to study this." Various areas such as infectious disease treatment and life support devices are emerging as candidates for essential medical devices, and Lee said, "The concept of essential medical devices is expected to be similar to that of the essential medicines. However, since there is no legal definition, we will conduct a research service this year and hope to see visible results by next year.” Another rising issue for MFDS this year is the ‘Digital Medical Products Act,’ which was enacted in January. The Digital Medical Products Act covers not only digital medical devices with digital technologies such as digital sensors and mobile applications, but also digital medical and health support devices that apply digital technologies for medical support and health maintenance and improvement, and digital convergence medicines that combine these devices and medicines. Under the act, a regulatory system for clinical trials, approval, and post-approval management has been established to efficiently and systematically evaluate the safety and efficacy of digital medical products based on digital characteristics such as the use of artificial intelligence and network connectivity. (From the left) Sang Hyun Kim, Director of the Medical Device Management Division, Nam-Hee Lee, Director-General of the Pharmaceutical Safety Bureau, Hong-Mo Sung, Director of the Medical Device Policy Division, Seung-Young Lee, Director of the Innovative and Diagnostic Medical Devices Policy Division Director-General Lee said, “We will prepare sub-statues so that digital medical products can be quickly commercialized. It will be beneficial to the industry if our law can be used to promptly provide devices to the public and will position Korea as a global leader." Last year, the digital therapy devices Somzz’ and Welt's ‘WELT-I’ were designated as the first and second digital therapy devices. However, it took more than 10 months from the approval to actual prescriptions, and it will take time for their reimbursement as the clinical outcome data has not been accumulated in hospitals. Director-General Lee said, “The Ministry of Health and Welfare is thinking about various ways to improve the system, and has first decided to designate products as innovative medical devices to enable integrated review. However, we are devising ways to enter the devices into the insurance system in some form, because reimbursement is possible only after the evaluation results of the product." However, when the next digital therapeutic devices, such as the third and fourth devices, are approved by the end of the year, Lee expected the innovative medical device designations to shorten the time to their prescriptions in the clinic first on a non-reimbursed basis. If it is designated as an innovative medical device, it will be subject to an expedited approval review, which currently takes more than 600 days on average. So I believe the clinical prescription period of the No. 3 product will be shortened further."
- Policy
- Liver cancer therapy, ‘Stivarga’ set for 2nd RSA renewal
- by Lee, Tak-Sun Mar 19, 2024 05:44am
- Stivarga. It has been reported that a risk sharing agreement (RSA) has been renewed for the second time for Stivarga (regorafenib; Bayer Korea), a second-line treatment for liver cancer. The news comes two months before the reimbursable RSA contract for Stivarga is set to expire on May 31st. According to industry sources on the 18th, the National Health Insurance Service (NHIS) agreed to renew a RSA with Bayer for Stivarga. Under the RSA, Stivarga is expected to receive reimbursement for five years until May 2029. Stivarga was first listed In June 2016 as reimbursable under RSA for the first time for treating gastrointestinal stromal tumor (GIST). Then, since May 2018, it has been reimbursable through RSA for a second-line treatment for hepatoma. In May 2020, Stivarga successfully received the first contract renewal. The contract duration is four years until May 31st, 2024. At the time of the first renewal, Stivarga’s list price was reduced by 7%. After the price cut in January 2022, the current maximum list price is KRW 303,868. It has not been confirmed whether it will receive a price cut in the list price in this contract renewal. However, it is anticipated that there will be a change in the actual price. Stivarga is a second-line treatment for liver cancer, used after Nexavar, the first-line treatment. Its out-patient prescription amount was KRW 9.3 billion won, down 24% from the previous year. Overall sales of existing targeted anticancer therapies are slowing down as immunotherapy with reimbursement emerged. According to Real World Data, which involved 182 Asian patients (Korea, China, and Taiwan), median Overall Survival (OS) with Stivarga was 16.3 months, an improvement of about 54% compared to the previous Phase 3 clinical study, ‘RESORCE.’
- Policy
- Sotyktu, Adtralza set for reimbursement listing
- by Lee, Tak-Sun Mar 18, 2024 05:49am
- Sotyktu. Two drugs, plaque psoriasis drug 'Sotyktu (deucravacitinib)' and atopic dermatitis drug 'Adtralza (tralokinumab)' have completed the negotiation with the National Health Insurance Service (NHIS) and may soon be listed for reimbursement. Additionally, the upper price limit of Soliris (eculizumab) is expected to be reduced following the expansion of reimbursement for neuromyelitis optica. According to industry sources on the 15th, Sotyktu and Adtralza concluded negotiations with the NHIS to receive reimbursement. Sotyktu is the first TYK2 inhibitor to be approved in South Korea for treating adult patients with moderate to severe plaque psoriasis. It is administered orally once a day for convenience. The drug was approved by the Ministry of Food and Drug Safety (MFDS) last August and cleared the review by the Drug Reimbursement Committee of the Health Insurance Review and Assessment Service (HIRA) in December. BMS Pharmaceutical, the company for Sotyktu, agreed to the evaluation price suggested by the Drug Reimbursement Committee. Notably, the company accepted an amount below the standard for negotiation exemption, solely negotiating for the anticipated claim amount. BMS Pharmaceutical and Yuhan recently made a joint promotion agreement for Sotyktu. When the drug gets listed on reimbursement next month, Sotyktu will likely be distributed through the marketing network of Yuhan-BMS. Adtralza. LEO Pharma’s 'Adtralza' is a treatment for atopic dermatitis with an underlying mechanism of neutralizing interleukin-13 (IL-13). Adtralza approval is expected to expand the treatment options for atopic dermatitis as Dupixent (dupilumab) is the only biologic available for treating the disease. Like Sotyktu, Adtralza is expected to be listed for reimbursement after one year of approval. It was approved last August and cleared for review by the Drug Reimbursement Committee in November Adtralza began negotiations with the NHIS in last November and it has recently come to an agreement. Currently reimbursed for atypical hemolytic uremic syndrome (aHUS) and paroxysmal nocturnal hemoglobinuria (PNH), Soliris is expected to receive approval for additional reimbursement for neuromyelitis optica spectrum disorder (NMOSD). Soliris is under pre-review by the HIRA. It is reported to be a high-priced new drug, valued at approximately KRW 500 million. Since 2015, the financial burden of the drug has been mitigated through Risk Sharing Agreements (RSA) but transitioned to regular listing in October 2019. Soliris. The company submitted the request for expanding Soliris’ reimbursement to neuromyelitis optica in 2021, but it took some time to be listed for reimbursement. After concluding negotiations with the NHIS, the coverage by reimbursement is expected to begin next month. With the reimbursement expansion, the current upper price limit of KRW 5,130,000 is expected to be reduced.