- LOGIN
- MemberShip
- 2025-12-21 03:55:05
- Policy
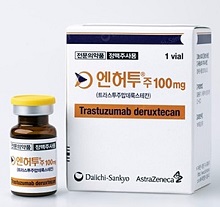
- Enhertu’s reimb to be redeliberated at next DREC meeting
- by Lee, Tak-Sun Jan 12, 2024 07:05am
- Reimbursement for the new breast cancer drug ‘Enhertu Inj,’ which was first deliberated by the Drug Reimbursement Evaluation Committee, will be redeliberated at next month's DREC meeting. At the same meeting, JW Pharmaceutical’s iron deficiency treatment ‘Ferinject Inj (ferric hydroxide carboxymaltose complex) was deemed adequate for reimbursement and moved on to conduct pricing negotiations with the National Health Insurance Service. The Health Insurance Review and Assessment Service said on the 11th that it deliberated as above at its 1st Drug Reimbursement Evaluation Committee meeting in 2024 (DREC). At the meeting, DREC deliberated on the reimbursement adequacy of ‘Ferinject Inj’ and ‘Enhertu Inj 100mg.’ Deliberation results of 1st DREC meeting in 2024 The reimbursement adequacy of Enhertu Inj 100mg (trastuzumab deruxtecan, Daiichi Sankyo Korea), which attracted attention as a drug that was having difficulty passing deliberations due to its high cost despite its high effect, was not concluded at this meeting and will be redeliberated at the February meeting after the company submits an improved financial sharing plan. The drug is indicated for ▲HER2-positive breast cancer and ▲HER2-positive gastric or gastroesophageal junction (GEJ) adenocarcinoma. On the other hand, Ferinject Inj, which is used to treat iron deficiency, was deemed adequate for reimbursement. Meanwhile, ‘Lorviqua Tab 25, 100mg (lorlatinib, Pfizer Korea),’ which applied for expanded scope of use as a Risk-Sharing Agreement drug, was deemed to be eligible for the expanded reimbursement for anaplastic lymphoma kinase (ALK)-positive metastatic non-small cell lung cancer if the company accepts a price below the evaluated value.
- Policy
- HIRA establishes new Pharmaceutical Benefits Dept
- by Lee, Tak-Sun Jan 11, 2024 05:44am
- High-priced drugs are changing the drug expenditure review paradigm in Korea. The high cost borne from the use of high-price drugs is increasing the importance of post-listing evaluation of high-priced drugs. To address this shift, the Health Insurance Review and Assessment Service established a ‘Pharmaceutical Performance Evaluation Department’ earlier this year, which is in charge of the post-listing management of high-priced drugs. This is the first time another pharmaceutical department has been established in HIRA since the Pharmaceutical Benefits Department was separately established from the Benefits Listing Department in 2006. According to industry sources on the 9th, HIRA had newly established a Pharmaceutical Performance Evaluation Department under its Health Insurance Review and Assessment Research Institute through restructuring earlier this year. The Pharmaceutical Performance Evaluation Department will be led by Director-General So-young Lee, who also serves as the Head of the Health Insurance Review and Assessment Research Institute. Lee graduated from Chung-Ang University College of Pharmacy and has a strong background in pharmaceutical listing management, including experience as the Director-General of HIRA's Benefits Listing Department. Also, Director Mi-Kyung Kim was appointed to the Pharmaceutical Performance Evaluation Department, who returned after receiving education at the Seoul National University School of Public Health. The Pharmaceutical Performance Evaluation Department will take over duties of the New Drug Performance Management Department that was established as a temporary organization under the Pharmaceutical Benefits Department in September 2022. That department had been temporarily established to address the need for post-listing management of high-priced drugs in line with the reimbursement listing of Kymriah, which costs KRW 360 million per dose. Specifically, the new department will be responsible for evaluating and analyzing the outcomes of patients receiving high-priced drugs at medical institutions. The number of items that are subject to performance evaluations has increased to 4, and includes Kymriah Zolgensma, Spinraza, and Evrysdi. Kymriah (above) and Zolgensma, drugs receiving post-marketing evaluation as high-priced drugs The Drug Performance Evaluation Department currently consists of 9 people under the Director-Genral and plans to expand its workforce in the future. For now, it is in charge of evaluating the performance of high-priced drugs, but in the future, it is expected to serve as a window for the post-listing management of PE exemption drugs that waived submission of pharmacoeconomic evaluation data. HIRA’s webpage introduces the duties of the Pharmaceutical Performance Evaluation Department as ▲ matters related to preparing the basis for performance management of high-priced drugs and system operations ▲ matters related to RWD utilization (E-form, etc.), and post-listing management ▲ matters related to cost-effectiveness evaluation and economic evaluation (reevaluation) of high-priced drugs ▲ matters related to research on the price, listing, and usage of drugs subject to performance evaluations. The post-listing management PE exemption drugs were also mentioned in the recently concluded report on ''Measures to improve the PE exemption system.' In the report, the researchers said, "The economic feasibility of listed PE exemption drugs were evaluated in all other HTA-based major countries,” suggesting the need for reevaluation of these listed drugs for the proper management of the price of drugs that will potentially be used as comparators for the listing of latecomers. The need for post-listing evaluation of PE exemption drugs based on RWE data was also mentioned during a public hearing on 'Measures to Manage Performance-based Reimbursement of Drugs using RWD/RWE' that was held in November last year. Ji-hye Byun, an associate researcher who published the RWE guideline for performance-based reimbursement management, is also in the Drug Performance Evaluation Department. When the plan to improve the post-management of PE exemption drugs is prepared, which is being promoted in earnest this year, the Pharmaceutical Performance Evaluation Department is likely to take the lead and take over the post-listing management work. The establishment of the Pharmaceutical Performance Evaluation Department holds significance as it serves as proof that the drug review paradigm has been changing. HIRA’s Pharmaceutical Benefits Department was separately established from the Benefits Listing Department in 2006 in response to the need to strengthen the management of drug prices and drug expenses. At the time, the measure was made in preparation for the positive-list system set to be introduced in December of the same year. If the positive-list system, which selects and applies reimbursement benefits to only clinically and economically high-value drugs, has led to changes in drug management work, it is interpreted that the new department opened a new paradigm in drug management that complements areas missed by the positive-list system, extending its work to post-management of high-value drugs. in his New Year's speech, Jung-Gu Kang, Director of HIRA, said, “Reimbursement for ultra-high-priced drugs and rare and incurable diseases has been rising as an ongoing issue recently. The entry of rapidly listed ultra-high-priced drugs into Korea’s reimbursement system after waiving economic evaluation has raised the need for post-management of drugs.” He added, “We want to create a mechanism that can monitor the treatment performance of a drug on each patient so that effective drugs can be administered to the public, and also reduce the risk of wasting major health insurance financial expenditures by strengthening post-listing management of drugs based on performance management."
- Policy
- MFDS to resolve production of chemotherapy drug '5-FU'
- by Lee, Hye-Kyung Jan 10, 2024 05:42am
- MFDS resolves One of the domestic pharmaceutical companies has agreed to produce the chemotherapy drug ‘5-fluorouracil (5-FU) injection.’ This measure will significantly aid in the treatment of cancer patients. ‘Since November last year, we received an information from the Korean Society of Health-system Pharmacists about a drug shortage of the chemotherapy drug 5-FU, and we reached out to the manufacturing company to investigate the cause,’ Kim Seonyoung, an official from the Ministry of Food and Drug Safety (MFDS)’s Heath Insurance Policy Review Committee stated. JW Pharmaceutical is responsible for the distribution of 5-FU, with Il Dong pharmaceutical serving as the Contract Manufacturing Organization (CMO). The MFDS discovered that Il Dong Pharmaceutical's efforts to upgrade certain facility units disrupted the production schedule of 5-FU. “To facilitate the drug’s distribution, we have expedited the production schedule, including stages like product quality testing and post-manufacturing processes. We have confirmed successful distribution of the drug on January 4th,” Official Kim stated Furthermore, another domestic pharmaceutical company has agreed to starting production of 5-FU as soon as the active ingredient is secured. This is expected to significantly improve the efficiency of the drug’s distribution starting from next month. Only one domestic pharmaceutical company is currently responsible for the distribution of 5-FU. To prevent any potential distribution instabilities, the committee inquired with several domestic pharmaceutical companies about their ability to produce the drug,” Official Kim mentioned. “With one of these companies now committed to distribution, more efficient distribution is likely starting in February.” The domestic pharmaceutical company that has agreed to produce 5-FU has requested not to disclose its name publicly. The Korean Pharmacists for Democratic Society (KPDS) announced a statement in response to the news about the supply shortage of 5-FU, a drug that has been listed as a shortage prevention drug (SPD) since 2010, that began in Dec. 2023. “Cancer patients are facing delays in chemotherapy schedules by 1 to 4 weeks or are frequently switching to alternative drugs,” the KPDS criticized. “The government claims to have a system in place for monitoring the supply and demand of essential medicines and receiving company reports on supply halts, in collaboration with relevant agencies and experts. However, details regarding the government's actions and corrective measures are lacking.”
- Policy
- Will Enhertu be discussed in this year’s 1st DREC meeting?
- by Lee, Tak-Sun Jan 10, 2024 05:42am
- The schedule for the Health Insurance Review and Assessment Service’s Drug Reimbursement Evaluation Committee meetings in 2024 has been finalized and released. The committee meeting will be held at the beginning of each month, starting with the first meeting on the 11th of this month. According to industry sources on the 8th, the 2024 DREC meeting schedule has been finalized. It will be held at the start of each month - on the 11th of this month, the 1st of next month, the 7th of March, the 4th of April, and the 2nd of May. At this month's meeting, it will be interesting to see if the new breast cancer drug Enhertu, which has been struggling at the pharmacoeconomic evaluation stage, will be presented for deliberation. Although the drug offers a significantly improved effect over existing drugs, it has faced difficulties in pharmacoeconomic evaluations due to its prolonged administration period. Therefore, when the government recently announced that the ICER threshold will be flexibly operated for innovative new drugs in the 'Innovative New Drug Appropriate Value Recognition Plan', Enhertu was considered to become a beneficiary. However, the plan to recognize the fair value of innovative new drugs has not yet been implemented. If Enhertu’s reimbursement agenda successfully passes the DREC review, it will not have to wait for the new system to settle into the country. Last month, Enhertu reportedly submitted supplementary pharmacoeconomic evaluation data to HIRA. In addition, an application for a price increase adjustment for the pediatric cough expectorant tulobuterol will be reviewed by the committee. Tulobuterol is facing difficulties in supply and demand due to the discontinuation of the original product, the increase in the unit cost of raw materials, and the increase in pediatric respiratory diseases. In response, the government plans to increase the drug price to encourage production. 2024 DREC Meeting Schedule The 2024 reimbursement adequacy reevaluation plan is also expected to be discussed in the February meeting. The first results of the reevaluation are expected to be presented to DREC in its August meeting and the final results at the December meeting. In addition, the reevaluation of sodium hyaluronate eye drops, which was not decided last year, is also expected to be redeliberated and reported to the DREC within the year. In addition, it is analyzed that the reevaluation of drug prices through external reference pricing, and the pharmacoeconomic evaluation data waiver system among others, may be discussed at this year's meeting.
- Policy
- Data Exclusivity Bill for IMDs may soon be legislated
- by Lee, Jeong-Hwan Jan 09, 2024 05:49am
- A bill to amend the Pharmaceutica Affairs Act, which grants a 6-year data exclusivity for incrementally modified new drugs that have obtained domestic marketing authorization, was reviewed during the plenary session of the Legislation and Judiciary Committee that was held on the afternoon of the 8th. If approved by the committee, the bill will be passed during the plenary session tomorrow (Sept. 9) and be legislated. The bill also suggest abolishing the drug re-review system and replacing it with a risk management plan (RMP). In particular, there is great interest in the new provision in the bill that recognizes exclusive rights to new and incrementally modified drugs and protects clinical trial data submitted at the time of drug approval. Specifically, the bill provides 10 years of protection from the date of approval for orphan drugs. This can be extended by an additional year if a pediatric indication is added. New drugs are granted 6 years of data exclusivity from the date of approval, and drugs that submit new clinical trial data, such as cto hanging the type of active ingredient to improve the safety, efficacy, and utility of already approved drugs, are granted 6 years of exclusivity from the date of approval. Based on this provision, IMDs can be granted a 6-year data protection period based on the provision. In addition, drugs designated by the Prime Minister's Decree that require submission of new clinical trial data are granted a 4-year data protection period. The existing reexamination system provides 10 years of data protection for orphan drugs, 6 years for new drugs and drugs with new active ingredients, formulation ratios, and routes of administration, and 4 years for new drugs with new efficacy and effect. If the bill passes the National Assembly, it is expected the data exclusivity of IMDs developed many domestic pharmaceutical companies will be recognized, Teh bill provides exclusivity when developing improved versions of their existing drugs, especially in terms of fficacy or utilit, with raises possibility of the development of a cash cow during new drug development. The bill is likely to pass the National Assembly because the Ministry of Food and Drug Safety (MFDS) is in favor of it and has been involved in its legislation, and it is in line with the government's policy of creating domestic blockbuster drugs. The MFDS said, "We agree with the purpose of the amendment to reduce the burden of data submissions to the pharmaceutical industry by integrating the drug reexamination system and risk management system, and to enhance Korea R&D capabilities in the pharmaceutical industry by establishing a legal basis for the drug data protection system."
- Policy
- Need to specify conditions for postponing PE data submission
- by Lee, Tak-Sun Jan 05, 2024 05:41am
- Study on Improving the Pharmacoeconomic Evaluation Data Waiver (PE exemption) System The results of the research service that was ordered by the Health Insurance Review and Assessment Service to devise measures on improving the pharmacoeconomic data submission waiver system, or the PE exemption system, have been disclosed in full. The improvements suggested by the researchers include redesigning the PE exemption system into a PE deferral system, and establishing a process to demonstrate economic feasibility after listing. It was also suggested that a reevaluation system should be established for drugs that have already been listed through the PE exemption track. Based on the study, HIRA plans to come up with a follow-up and reevaluation plan for the PE exemption drugs. According to the results of the ‘Study on Improving the PE evaluation data waiver system (Seoul National University R&DB Foundation, Professor Tae-jin Lee, Principal Investigator)' that was released on the 3rd, ithe PE exemption system needs to be redesigned into a system that defers proof of economic feasibility for drugs when necessary, rather than a system for waiving submission of PE evaluation data overall. The PE exemption system was introduced in May 2015 and applied to 26 drugs until July 2022. Explaining the background of their proposal, the researchers said, "The introduction of the PE exmpetion system has had some positive effects on improving patient access, such as by improving the rate of new drug listings and shortening the listing period. However, when considering the various characteristics of subject drugs, there seems to be a high need for the PE exemption drugs to be managed within the basic principles of the positive listing system that is based on the demonstration of cost-effectiveness.” In this regard, the research team proposed ▲ redesigning the system into an economic feasibility demonstration deferral system ▲ establishment a post-listing economic feasibility demonstration process through a specific agreements on items that need to be clarified in advance ▲ establishment of a post-marketing and reevaluation system based on cost-effectiveness evaluations ▲ and others, such as increasing the practicality of total expenditures by setting a set amount for each disease unit or an expenditure cap and setting a reasonable baseline for the evaluation amount based on foreign drug price. The researchers also saw the need to reestablish the current PE exemption conditions. More specifically, the condition, ‘anticancer drugs or rare diseases for which no substitute or therapeutically equivalent product or treatment exist,' needs to be specified. On this, the researchers proposed limiting the condition to ‘rare disease drugs or anticancer drugs used for serious conditions that threaten survival, such as those for diseases with a life expectancy of less than 2 years,’ and to set specific requirements such as ‘drugs that bring a significant clinical improvement over nontreatment and has no other alternative treatment,’ or ‘provide a significant clinical improvement over existing treatments, such as a significant prolongation of survival.’ The reseachers also believed that the current grounds for lacking evidence were also inadequate. The current requirement is: drugs for a small number of patients, ▲ that were approved with single-arm clinical data without a control group, ▲ approved with a Phase II clinical trial that has a control group but without a conditional Phase III trial, or ▲ have other difficulties in producing evidence. More specifically, the researchers proposed ▲ cases where no clinical trial with a control group has been conducted and indirect comparison is difficult, ▲cases where PE evaluations have not been conducted in other countries as well, ▲cases where it is difficult to confirm the final effect due to immature clinical data, and it is not appropriate to conduct PE evaluation by estimating the final result through modeling. However, ▲if the size of the required finances is above a certain level, economic feasibility must be demonstrated. Also, the researchers pointed out that the requirement of a ‘small number of patients’ should also be revised to a ‘diseases with a prevalence 200 or less patients per independent condition.' In addition, the researchers claimed that drugs used for pediatric patients and tuberculosis treatments, which were added as PE exemption drugs last year, should be removed again as drugs that are not considered to be rare diseases but are life-threatening were waived PE data submissions even before the revisions were made. The researchers concluded that real-world evidence (RWE) can be recognized as a source of evidence after listing. They also suggested the need for a collective reevaluation of the listed PE exemption drugs. "In the case of PE exemption drugs they have already been listed, all other HTA-based countries have evaluated their economic feasibility after listing. Therefore, it is necessary to reevaluate the drugs even if their control period has been completed to manage the appropriate listing price of latecomers that will be listed using the PE exemption drugs as a comparator."
- Policy
- Moderna produced the most drugs in Korea in 2023
- by Lee, Hye-Kyung Jan 04, 2024 05:33am
- Moderna Korea has surpassed Celltrion and Hanmi Pharmaceutical and became the largest domestic pharmaceutical manufacturer in Korea in 2022. The item that drove Moderna’s lead was the COVID-19 vaccine ‘Spikevax Inj,’, which accounted for 100% of Moderna Korea's total production value of KRW 1.275 trillion. According to the '2023 Food and Drug Statistics Yearbook' recently published by the Ministry of Food and Drug Safety, the top 20 domestic pharmaceutical companies in 2022 in terms of the amount of drug production are Moderna Korea, No. 1, Celltrion, No. 2, Hanmi Pharmaceutical, No. 3, Chong Kun Dang, and No. 5, GC Biopharma. Hanmi Pharmaceutical, which ranked first in 2018 and 2019, and Celltrion, which ranked first in 2020 and 2021, lost the top spot to Moderna Korea in 2022 due to the rise in supply of vaccines amid the spread of COVID-19. Among the top 20 pharmaceutical companies in Korea, the companies with the highest manufacture amount exceeding KRW 1 trillion were Moderna Korea (KRW 1.275 trillion), Celltrion (KRW 1.226 trillion), Hanmi Pharmaceutical (KRW 1.018 trillion), and and Chong Kun Dang (KRW 1.594 trillion). Two dosage forms of Spikevax were ranked first and second in terms of production of single products, followed by Celltrion's Remsima 100mg at KRW 184.9 billion, Handok's Plavix Tab 75mg at KRW 153.4 billion, and HK Inno.N's K-Cab 50mg at KRW 152.3 billion, Daewoong Pharmaceutical's 'Nabota' at KRW 127 billion, GC Biopharma’s 'GC Fluquadrivalent Prefilled Syringe Inj' at KRW 116.6 billion, and Chong Kun Dang’s ‘Chong Kun Dang’s Gliatorin Soft Cap' at KRW 107.9 billion, among drugs with an annual production amount that exceeds KRW 100 billion for a single item. In particular, although reimbursement was reduced due to reimbursement revaluations for choline alfoscerate, the production of Chong Kun Dang’s Gliatorin Soft Capsule and Daewoong Bio's Gliatorin Soft Capsule (KRW 97.2 billion), representative choline alfoscerate products, still ranked 8th and 9th in Korea. Among the top 20 global pharmaceutical companies, in 2022, AbbVie ($73 billion) ranked first, followed by Johnson & Johnson ($71 billion), then Novartis ($57 billion), Novo Nordisk ($50 billion), then Bristol-Myers Squibb ($49 billion). The top 10 finished drug imports for 2022 were also listed, with Comirnaty Inj Veklury Intravenous Lyophilized Powder for Injection ranking No. 1, followed by Comirnaty 2 Injection 0.1mg/m, Spikevax Inj, Keytruda Inj, then Prolia Prefilled Syringe ranking the fifth.
- Policy
- Comprehensive Health Insurance Plan to be released soon
- by Lee, Jeong-Hwan Jan 03, 2024 05:40am
- With the government's plan to announce the 2nd National Health Insurance Comprehensive Plan being postponed from December last year to January of this year, the pharmaceutical industry is anxiously awaiting what policy direction will be included in the new plan regarding drug expenditures. The pharmaceutical industry’s wishes are that the plan should include policies to promote and revitalize domestic generics and incrementally modified drugs that can serve as cash cows in securing research and development (R&D) investment costs for new drug development. On Jan. 1, the Ministry of Health and Welfare is known to be in the final stages of formulating the second health insurance plan, which will be in place for 5 years from this year (2024) to 2028. The MOHW had aimed to release the plan in December last year, but postponed the announcement, saying it needed more time to reflect the newly announced policies. This implied that the MOHW was not finished organizing the policies it had finalized last year, such as setting a public policy fee to support essential healthcare and the reform of the drug pricing system to reflect innovation value, into the comprehensive plan. The industry’s eyes are on the drug expenditure management direction that will be included in the new health insurance plan. In particular, domestic pharmaceutical companies are concerned that if the MOHW includes a new mechanism for reducing drug prices in addition to the post-listing reimbursement management of generic drugs that are already in place, this will dampen the industry’s drive for new drug R&D. In the study on the establishment of a comprehensive health insurance plan released by the Korea Institute for Health and Social Affairs in October last year, no mention had been made of the need to introduce a new drug price reduction model for generic drugs. However, the study suggested a policy to expand the targets subject to reevaluation for drug reimbursement adequacy review and to reevaluate generics by comparing their drug prices with the overseas A8 countries (Japan, France, Germany, Italy, Switzerland, the United Kingdom, the United States, and Canada). It also revealed plans to improve the effectiveness of the actual transaction price investigation and management system, reorganize the targets of the Price-volume Agreement system, and revise its formula. Furthermore, the MOHW had set criteria for easing the application of the PVA system for drugs made by innovative pharmaceutical companies or equivalent in the reform plan for the drug pricing system that recognizes the innovation value of drugs. Despite such improvements, domestic pharmaceutical companies are nervous as the MOHW is still known working on a drug price reduction model for generics. A domestic pharmaceutical company official said, "We hope that the new health insurance plan will not include a new price reduction mechanism. We also need to up with a reasonable reorganization plan for the post-listing management measures that are already in place. Also, we would like to discuss the permanence of the drug price reduction dispositions." The official added, "Although a drug pricing system that reflects innovation value has been introduced, it is partly focused on new drugs or essential drugs with low monetization. We need more measures to encourage generic development, which is the source of cash generation, to strengthen Korea’s new drug development momentum.” Another domestic company official said, "I hope that the MOHW will hold a closer ear to the voices of companies with the aim of advancing post-management mechanisms including the PVA system. In the big picture, I hope that the new plan will include short- and long-term policies to save health insurance finances and revitalize the pharmaceutical market to exceed the performance made this year.”
- Policy
- Subject drugs with claims over KRW 5 bil for price nego
- by Lee, Tak-Sun Jan 03, 2024 05:40am
- A study has shown that more drugs need to be subject to PVA negotiations due to the expanded scope of use. In other words, the current standard, which sets the expected additional claims amount in the KRW 10 billion range needs to be expanded to KRW 5 billion, and drugs with an expected increase rate of more than 100 % should also be subject to negotiations. Such results were disclosed as part of the 'Study on the Performance and Improvement of the Scope of Use Expansion Negotiation System' that was conducted as a research service for the National Health Insurance Service (Research Institute Yonsei University, Industry-Academia Cooperation Center, Professor Euna Han). The research team said, “The current system was introduced in 2014 and has been maintained for 10 years without any revision. With the continued rise in national health insurance’s drug expenditures and the increasing number of drugs with multiple indications, many of which are expensive RSA drugs, there is a clear need for revision of the system.” The research team said, "It is important to review the performance of the expanded use negotiation system, which proactively manages drug costs for expanded use of drugs, represented by expanded indications, and to prepare development plans to strengthen the capability of the system,” and suggested short- and long-term improvement measures. As a short-term improvement plan, the researchers first proposed that the criteria for the selection of negotiation targets need to be expanded from the current estimated additional claims amount of KRW 10 billion or more to an estimated additional claims amount of KRW 5 billion or more and to establish new criteria for selection of negotiation targets based on the estimated additional claims amount (e.g., an increase of 100% or more). Secondly, as a measure to improve the performance of the negotiation system, the researchers proposed that the reduction rate set through prior adjustments should be increased, which is considered one of the price criteria used during negotiation. In addition, the researchers also proposed differentiated drug price cuts based on the rate of increase in claims, not by just the additional claims amount. In the long term, the researchers proposed that drugs subject to focus management (e.g., drugs with annual claims of more than KRW 30 billion or annual expenditures of more than KRW 300 million per person) should be selected and be designated as mandatory negotiation targets when expanding their scope of use, regardless of the selection criteria. Second, although the current system that manages the expanded scope of use uses the period one year before and after the expansion of use as the reference period, considering that the amount of usage and claims for one year after the expansion may differ from the use amount and claims made in the mid-to-long-term. Therefore, the researchers requested the government to consider post-management measures such as financial impact assessment and drug price adjustment measures accordingly from the mid-to-long-term perspective. The researchers also suggested strengthening the linkage with the Price-Volume Agreement system in the short term to minimize the loss in the monitoring period arising from the expansion of the scope of use and in securing the sustainability of health insurance by strengthening reimbursement management.
- Policy
- 430K osteoporotic fractures in 2022, a steep rise over 20 yr
- by Lee, Tak-Sun Jan 02, 2024 05:45am
- There has been a yearly increment of 7.8% in patients with fractures due to osteoporosis, marking a staggering 346% increase compared to 20 years ago. Joint research conducted by National Health Insurance Service (NHIS) (Chariman: Jung Ki Suck) and The Korean Society for Bone and Mineral Research (Chariman: Ha Yong-Chan) has presented research findings titled ‘Reporting the incidences of osteoporotic fractures and re-fractures among Koreans over 50 years old,’ based on the National Health Insurance Big Data for the periods of 2002 to 2022. The overall incidence of osteoporotic fracture totaled 434,470 patients in 2022, a 34.2% increase compared to 323,806 patients in 2012, and a 346.2% increase compared to 97,380 patients in 2002. Additionally, it shows a yearly increment of 7.8%. Over the last 20 years, the overall incidence of osteoporotic fractures has steadily increased, with a yearly average rate of 8.1% increase in men and a 7.6% increase in women. A yearly rate of osteoporotic fracture incidences based on the National Health Insurance Big Data for the periods of 2002 to 2022. There has been a yearly increment of 7.8% in patients with fractures due to osteoporosis, marking a staggering 346% increase compared to 20 years ago. When considering gender differences, the prevalence of fracture was 3.1 times higher in women (329,104 people) than men (105,366 people). Among men, 29.1% were in their 60s, while 33.1% of women were in their 80s. (Unit: n, %) As of 2022, out of a total of 434,470 fracture patients, 31.0% were in their 80s (134,549), 26.3% were in their 70s (114,273), 26.4% were in their 60s (114,886), and 16.3% were in their 50s (70,762), demonstrating a steep increase with advancing age. When considering gender differences, the prevalence of fracture was 3.1 times higher in women (329,104 people) than men (105,366 people). Among men, 29.1% were in their 60s, while 33.1% of women were in their 80s. Wrist and ankle fractures were common in the ages of 50s to 60s, and the number of vertebral and hip joint fractures increased with age. The 1-year mortality rate for patients with hip joint fractures exhibited a decreasing trend from 18.9% in 2006 to 15.9% in 2020 but increased again to 18.2% in 2021. In contrast, the 1-year mortality rates of hip fracture or vertebral fracture remained without any changes over the years up to 2020, but saw an increase in 2021, presumably due to the effects of Covid-19. During the last 20 years, the rate of osteoporotic fracture patients receiving osteoporosis-treating medications within a month is 22.0% in 1 month, 28.9% in 3 months, 32.2% in 6 months, and 35.5% within a year. In terms of medications, the highest prescription rate within a year following a fracture was for bisphosphonate class drugs 30.8%, followed by denosumab 3.3%, Selective estrogen-receptor (ER) modulators (SERMs) 2.9%, parathyroid hormones (PTH) 0.7%, and romosozumab 0.15%. The prescription rate for fracture treatments within a year following a fracture showed a steady increase for both men and women over the years. A 2.5 times higher proportion of women, with a rate of 46.9%, started treatments within a year compared to men, with a rate of 18.7%. In terms of fracture localizations, the highest prescription rate within a year following a fracture was the vertebral fracture with 52%, and the lowest was the ankle fracture with 15%.