- LOGIN
- MemberShip
- 2025-12-21 05:47:55
- Policy
- Bill pending on substituting dispositions for illegal rebate
- by Lee, Jeong-Hwan Dec 20, 2023 05:40am
- A bill that would allow companies to substitute the price cut/rebate suspension dispositions made on their respective drugs due to rebates has been put on hold at the Standing Committee’s Legislation and Judiciary Subcommittee stage. The review for the bill continued after the Ministry of Health and Welfare, the ministry in charge of the bill, turned down the subcommittee members’ suggestion that the current law should be amended to eliminate cases where third parties – such as patients or medical institutions - are harmed by the reimbursement suspension dispositions. As a result, the bill would need to pass the Health and Welfare Committee's subcommittee review before next year's general elections to be enacted during the 21st National Assembly's session. On the 19, the 2nd Legislation and Judiciary Subcommittee of the National Assembly's Health and Welfare Committee reviewed the partial amendment to the National Health Insurance Act presented by Representatives Min-Seok Kim of the Democratic Party of Korea and Jong-Seong Lee of the People’s Power Party. The subcommittee members determined that the bill should be further reviewed at the subcommittee stage and gave the agenda a pending ruling. The bill sought to improve the unreasonableness of administrative dispositions that require patients to change their existing medications to other drugs, such as the suspension of reimbursement imposed on rebate drugs. In particular, the bill includes a retroactive provision to replace administrative penalties with fines for drugs that provided rebates before the amendment of the Health and Welfare Act in 2018. During the subcommittee meeting, Rep Min-Seok Kim, who presented the bill as a representative, said to the MOHW, "Do you really believe there is no room for change in the current system? The original administrative penalty regulation imposes penalties on innocent patients rather than the errant pharmaceutical companies." Therefore, Kim’s argument is that the Health Insurance Act should be amended to allow for penalties to be substituted for fines to prevent unnecessary confusion in medical institutions and damage to patients. However, due to the MOHW’s nonacceptance, the bill was unable to pass the subcommittee review. MOHW Deputy Minister Min-soo Park said, “We already exclude reimbursement suspension dispositions for drugs that have no alternatives. Also, the reimbursement suspension dispositions are not permanent. Although re-registration may be difficult, this is more of an implementation problem in the field. We will negotiate with relevant stakeholders so that they are not punished more than what was purposed by the law.
- Policy
- Forxiga generics continue being released in the Korean mkt
- by Lee, Tak-Sun Dec 20, 2023 05:40am
- Generic versions of Forxiga’s salt-modified drug, which were released in April, will be reimbursed from next month. These generics were released later than other Forxiga generics because the original salt-modified drug obtained first generic exclusivity in Korea, banning the sales of other salt-modified drugs that contain the same ingredient until January 7 next year. According to industry sources on the 19th, Foxiga’s salt-modified generics, including Samjin Pharamcetucial’s Dapozin Tab 5mg, Kyungbo Pharm’s Dapakhan Tab 10mg, will be listed next month Fixe single-agent and 8 combination drugs will be listed at the time. Samjin Pharamcetucial’s Dapozin Tab 5mg is a non-salt dapagliflozin 5mg product. Chong Kun Dang’s Exiglu Tab 5mg is the only other same-ingredient and dosage drug available with reimbursement in Korea, but, Chong Kun acquired the first generic exclusivity for its Exiglu upon release and banned other same-ingredient same-dose drugs from being sold in the market until January 7 next year. To avoid this, Samjin Pharamcetucial released a different dosage of the same ingredient drug, Dapozin Tab 10mg, in April this year. Kyungbo Pharm’s Dapakhan Tab 10mg is a 15.27mg dapagliflozin anhydrous lactose mixture. The 5 other generics with the same ingredient had obtained first generic exclusivity at the same time. Therefore, the same-ingredient same-dosage formulation drugs like Dapakhan 10mg can only be sold from January 8th next year. In addition to Dapakhan 10mg, Guju Pharma’s ‘Dafarizin Tab. 10mg,’ Unimed Pharm’s ‘Sugapa Tab. 10mg,’ and Pharmgen Science’s ‘Daflozin Tab 10mg’ will also be listed for reimbursement on January 8th. With the exception of Daflozin Tab 10 mg, whose company decided to select a price, the other four single-agent drugs received a 59.5% markup to the highest price of the same ingredient drugs. However, the premium pricing is only applied temporarily for 3 months as their price will be adjusted in line with the price adjustment of the original salt-modified drug on April 8 next year. In the case of combination drugs, new salt-modified combination drugs will be listed for the first time. Eight items, including Sinil Pharm’s ‘Forxigly Duo ER Tab 10/500mg', will be reimbursed from next month. Forxigly Duo ER Tab 10/500 mg contains 15.27 mg of dapagliflozin anhydrous lactose mixture. and 500 mg of metformin hydrochloride, and there is no other equivalent currently listed on the reimbursement list. Along with Sinil, HLB Pharm, Kyungbo Pharm, and Samjin Pharm will also be granted reimbursement for 2 different dosage forms of the combination each next month. Meanwhile, 64 Korean pharmaceutical companies have entered the Foxiga generic market so far.
- Policy
- New drugs Zeposia, Koselugo, Trimbow are reimbursed
- by Lee, Tak-Sun Dec 19, 2023 05:53am
- New ulcerative colitis drug .that will be reimbursed from next year New drugs including Zeposia Cap, Koselugo Cap, and Trimbow Cap will be listed for reimbursement starting January next year. In addition, Dong-A ST's diabetes combination drug ‘Sugatree XR Tab’ will also be reimbursed, and the reimbursement standards for drugs that have undergone reevaluation of their reimbursement adequacy will be adjusted as well. On the 18th, the Ministry of Health and Welfare announced that it has issued an administrative notice of the 'Partial Amendment of Details on the Application Standards and Methods of Medical Benefits' that contain the matters above, for enforcement as of January 1 next year. Zeposia Cap is used to treat moderate-to-severe active ulcerative colitis in patients who respond adequately to existing treatment or biological agents including corticosteroids, 6-Mercaptopurine, or azathioprine, or have no response, or have no resistance and are contraindicated to use such drugs. Trimbow Inhaler is used in adults (over 18 years of age) to treat moderate-to-severe chronic obstructive pulmonary disease (COPD) and asthma. In COPD, Trimbow is used for maintenance treatment in patients whose disease is not adequately controlled despite treatment with a combination of two medicines consisting of a long-acting beta-2 agonist plus either an inhaled corticosteroid or a long-acting muscarinic receptor antagonist. In asthma, Trimbow is used for maintenance treatment in adults whose disease is not adequately controlled despite treatment with a long-acting beta-2 agonist plus a medium or high dose of inhaled corticosteroid, and who had one or more exacerbations within 12 months. Koselugo is used conditionally for the treatment of pediatric patients over 3 years but 18 or below with neurofibromatosis type 1 (NF1) who have symptomatic, inoperable plexiform neurofibromas (PN). The patient’s PN is regarded inoperable if the lesion cannot be surgically completely removed without risk for substantial morbidity due to: encasement of or close proximity to vital structures, invasiveness, or high vascularity of the PN. Dong-A ST’s triple antidiabetic combination ‘Sugatree XR Tab’ will also be listed for reimbursement. The drug is a combination of metformin hydrochloride, dapagliflozin, and evogliptin. The MOHW added Sugatree’s combination to the list of dapagliflozin+sitagliptin+metformin combinations that were previously granted reimbursement as part of a metformin+SGLT2i+DPP4i regimen since April this year. Meanwhile, the reimbursement standards for drugs that have undergone reevaluation of their reimbursement adequacy in 2023 will also be adjusted at the same time. The results of the subject substances were presented at the Health Insurance Review and Assessment Service's Drug Benefits Evaluation Committee in July. The drugs for which reimbursement standards will be revised are loxoprofen sodium, limaprost alfadex, and epinastine hydrochloride. Loxoprofen’s antipyretic and analgesic indications for acute upper respiratory tract infections and limaprost alfadex’s indication for the treatment of ischemic symptoms of Buerger's disease will be removed from the reimbursement standards. In addition, the use of epinastine hydrochloride for bronchial asthma will be removed from the reimbursement list. Meanwhile, HA eye drops, which the government decided to continue discussions on its reevaluation results, will not be included in the upcoming reimbursement standard revisions.
- Policy
- PVA price cut rate will be raised by up to 15%
- by Lee, Tak-Sun Dec 18, 2023 05:31am
- From next year, items with higher insurance claims will be subject to higher price cuts when negotiating prices through the Price-Volume Agreement (PVA) system. The maximum price cut rate is also expected to be raised to 15% from the current 10%. However, the exclusion limit will also be raised to KRW 3 billion from the current 2 billion won, which is expected to increase the range of small and medium-sized products that benefit. The National Health Insurance Service reportedly held a public-private consultative body meeting with the Korea Pharmaceutical and Bio-Pharma Manufacturers Association and shared the plans above on the 15th. With this public-private consultation meeting as its last, the NHIS will prepare a final draft with the Ministry of Health and Welfare and apply the changes from January next year. However, changes requiring revision of notifications will be implemented after April next year. A change that does not require revision of the notification – changing the formula to be applied differently according to the claims amount – will be implemented from January next year. Based on the threshold of KRW 30 billion, a higher reduction rate will be applied for higher claims amounts. However, the range of items that are exempt from being applied PVA will be expanded. Currently, drugs with insurance claims of less than KRW 2 billion are excluded, but the plan is to raise this criterion to KRW 3 billion. In addition, a measure has been put in place to adjust the reduction rate or provide refunds for items with temporarily increased usage due to infectious disease situations or unstable drug supply, such as in the case of respiratory drugs that were sued for COVID-19. Furthermore, the innovative drugs mentioned will be recognized for their fair value, and the reduction rate will be lowered for innovative new drugs. If an innovative drug is subject to negotiation 3 times in 5 years, the discount rate will be reduced further during the third negotiation. The maximum reduction rate will be raised from the current 10% to 15-20%, with 15% being the most likely rate considering the range deemed acceptable by the pharmaceutical industry. The rate is expected to be implemented after April next year as it requires revision of the notification. The PVA improvement plan has been discussed with the pharmaceutical industry through a public-private consultative body after the results of the ‘A study on the performance of the Price-Volume Agreement System and measures on its improvement’ that Professor SeungJin Bae from Ewha Womans University's College of Pharmacy participated as the principal investigator, was released in April. The contents shared during the final public-private consultative body meeting were also based on the results of the research service. However, the final draft excluded the research service’s proposal of adding items whose claims had increased by 10% and exceeded by over KRW 5 billion in Type A of the PVA system, which the pharmaceutical industry responded most sensitively to.
- Policy
- Global orphan drug Lamzede receives GIFT designation
- by Lee, Hye-Kyung Dec 15, 2023 05:51am
- 'Lamzede Inj (velmanase)’ received the Global Innovative Products on Fast Track (GIFT) designation and will receive an expedited review in Korea. Lamzede is an orphan drug for which Kwang Dong Pharmaceutical signed an exclusive sales and distribution agreement with Italy's Chiesi Farmaceutical in July this year. As the GIFT program seeks to shorten the review period by at least 25%, the drug could be granted within 90 working days if Kwang Dong Pharmaceutical thoroughly submits the required supplementary materials. The Ministry of Food and Drug Safety recently announced that it had designated Lamzede, an enzyme replacement therapy for the treatment of non-neurological manifestations in patients with mild to moderate alpha-mannosidosis (AM) to receive review through the GIFT program. The MFDS grants the GIFT designation and the expedited review to ▲ drugs aimed at treating serious life-threatening diseases such as cancer or rare diseases ▲ drugs aimed at preventing or treating infectious diseases that may cause serious harm to public health, such as bioterrorism infectious diseases or pandemics, ▲ new drugs developed by Korea Innovative Pharmaceutical Companies designated by the Ministry of Health and Welfare, ▲ drugs used in combination with medical devices subject to expedited review, ▲ drugs that showed clinically significant improvements in effect over existing treatments or for which no existing treatment exists. Lamzede is a global orphan drug for which there are no existing therapies and was granted Priority Review (PR) by the US FDA on February 16, and was granted approval under the Marketing Authorisation Under Exceptional Circumstances (MAEC) program by the European Medicines Agency (EMA) in March 2018. Kwang Dong Pharmaceutical signed an exclusive domestic sales and distribution contract with the Italy-based global pharmaceutical company Chiesi Farmaceutical in July this year. Under the agreement, Kwangdong Pharmaceutical partnered with Chiesi Farmaceutici, for the exclusive sales distribution of Chiesi’s three rare disease drugs – Lamzede, Leber's neuropathy drug Raxone, and Fabry disease treatment Elfabrio – in Korea. The company plans to introduce Chiesi’s diverse lineup to the Korean market through the partnership agreement. In March, before partnering with Chiesi, the company had also signed an agreement with a Hong Kong opathamology drug company Zhaoke Ophthalmology to introduce the latter’s new drug candidate for pediatric myopia, 'NVK002,’ to Korea. Since the commencement of the program in September last year, a total of 23 products have been designated as GIFT products until now. Meanwhile, the first GIFT drug, Lunsumio (mosunetuzumab)’ has been approved in November this year. Drug subject to GIFT receives various support for the rapid commercialization of its product including at least a 25% reduction in the review period (ex: 120 working days → 90 working days), support for preparing approval data, rolling review support, and opportunities for close communication between the reviewer and developer and expert consulting on regulatory affairs, etc.
- Policy
- HER2-positive metastatic breast cancer drug Tukysa approved
- by Lee, Hye-Kyung Dec 15, 2023 05:51am
- The Ministry of Food and Drug Safety (MInister: Yu-kyoung Oh) approved two dosage forms (50mg, 150mg) of MSD Korea’s new breast cancer drug Tukysa (tucatinib) on the 14th. The drug is used in combination with trastuzumab and capecitabine, for patients with locally advanced or metastatic HER2-positive breast cancer who have received two or more prior anti-HER2-based regimens in the metastatic setting. Tucatinib is a tyrosine kinase inhibitor (TKI) that is a selective and potent inhibitor of the HER2 receptor overexpressed on cancer cells. It blocks the intracellular signaling pathway of HER2 to inhibit the survival, proliferation, and metastasis of tumor cells and induce cellular apoptosis The drug is expected to provide new treatment opportunities for HER2-positive patients who have been difficult to treat with existing therapies. MFDS said, "Based on our expertise in regulatory science, we will continue to make our best efforts to ensure that therapies with sufficiently confirmed safety and effectiveness are promptly supplied to expand treatment opportunities for our patients in Korea.”
- Policy
- Non-inferior drugs likely to receive preferential pricing
- by Lee, Tak-Sun Dec 15, 2023 05:51am
- Non-inferior drugs from Korea innovative pharmaceutical companies will likely be eligible for preferential drug pricing starting next year. Previously, non-inferior drugs were priced below the weighted average of alternative drugs. However, through improvement measures, if the patent of an alternative drug is still active, non-inferior drugs are now likely to be priced within a range between the weighted average price and the highest price of alternative drugs. According to the industry, the government and the pharmaceutical industry held a meeting for ‘Measures to Recognize the Appropriate Value of Innovative Drugs,’ and shared these updates. The improved measure states that if a drug of a Korea innovative pharmaceutical company receives approval through the Ministry of Food and Drug Safety (MFDS)'s GIFT system (Global Innovative Products on Fast Track, GIFT), it will be included and be applied the existing preferential treatment measures used during reimbursement review. The existing preferential treatment measures that will be used during reimbursement review for the GIFT drugs refer to the article, ‘1.7. Medicines in need of preferential treatment when considering its effects on public healthcare,‘ in the Health Insurance Review and Assessment Service (HIRA)’s ‘Specific evaluation criteria for new drugs and medicines subject to pricing negotiations.’ Specifically, the existing criteria contain preferential drug pricing measures for essential medicines endorsed by the WHO or national essential medicines, globally first-approved innovative drugs, and cell therapy products that satisfy the criteria. If the candidate drug’s clinical utility was similar to (or non-inferior to) an alternative, its price was set as ▲either an amount between the weighted average price and the highest price of the alternative drugs, or ▲the weighted average price of its alternative multiplied by (100/53.55), whichever was lower. The criteria has now been expanded to include drugs from Korea innovative pharmaceutical companies approved through the GIFT system. Whereas previously, non-inferior drugs from these companies were priced below the weighted average price if the alternative drug's patent had not expired, they can now be potentially priced at an amount between the weighted average price and the highest price of the alternative drugs. Since most new drugs developed domestically are non-inferior drugs from Korea innovative pharmaceutical companies, these homegrown new drugs will likely receive better drug prices in the future. Meanwhile, it has been reported that the agenda of including natural product-derived new drugs like cell therapy products as a subject to the preferential measures is set to be discussed in the future. Although this item was omitted from discussions in the meeting held on the 12th, it might be included again as an agenda for the Health Insurance Policy Deliberation Committee's subcommittee, which is scheduled to meet on the 14th. Additionally, it has been reported that for new drugs meeting the criteria for innovative drugs, there will be flexible application of the ICER (Incremental Cost-Effectiveness Ratio), and implementation of a risk-sharing plan to provide eased measures such as removing the final 3 negotiations if a drug has already received 3 three PVA negotiations during the past five years. The improvement measures are scheduled to be reported at the meeting of the Health Insurance Policy Deliberation Committee planned for the 20th, and the measures are expected to be sequentially applied from next year.
- Policy
- Will Enhertu be reimbursed using flexible ICER threshold?
- by Lee, Tak-Sun Dec 14, 2023 05:47am
- With the government reportedly planning to flexibly apply the Incremental Cost-Effective Ratio (ICER) threshold, whether the breast cancer treatment Enhertu (fam-trastuzumab deruxtecan, Daiichi Sankyo) will finally find its way to reimbursement is gaining attention in Korea. The drug’s reimbursement review is currently stuck in HIRA’s pharmacoeconomic evaluation stage. The drug was unable to pass the stage due to the longer period of administration, which rather increased drug cost, despite its significantly better effect than existing drugs. Therefore, a more flexible application of the ICER threshold during pharmacoeconomic evaluation is expected to allow Enhertu’s passage. According to the industry on the 13th, a plan to flexibly apply the ICER threshold for innovative new drugs in consideration of their innovative value was shared at the public-private consultative body meeting for 'Measures to recognize the appropriate value of innovative new drugs’ that was held on the 12th. The current ICER threshold is flexibly used to evaluate the cost-effectiveness of drugs in consideration of the severity of the disease, impact on quality of life, and innovativeness of a new drug. As the criteria used to evaluate the economic feasibility of a new drug with an improved effect, it determines the additional cost spent by a new drug compared to its comparator for the improved efficacy or each unit of use. If a drug’s ICER threshold is less than or equal to a certain level, the drug is interpreted as being cost-effective to its comparator. In general, the level is set at around KRW 50 million for anti-cancer and rare disease drugs and around KRW 30 million for other general drugs. Enhertu’s price had reportedly exceeded the ICER threshold of KRW 50 million. The industry believes that if the threshold is flexibly applied according to the characteristics of each drug, it could serve as a breakthrough for Enhertu’s reimbursement which is stuck in the pharmacoeconomic evaluation process. However, concerns have been raised about the strict standards set on which innovative new drugs may receive flexible application of the ICER threshold. To be eligible for flexible application of the ICER threshold, a new drug must meet all of the following criteria: ▲ has a new mechanism of action or substance, ▲ has no alternative treatments (including drugs) available, ▲ has shown clinically significant improvement, such as a significant extension in survival, ▲ received a Breakthrough Therapy Designation (BTD) from the U.S. FDA or approved as a Priority Medicines (PRIME) by the European Medicines Agency (EMA), and ▲ is a treatment for a rare disease or an anti-cancer drug. Enhertu must also meet all of the criteria above to receive preferential treatment in its pharmacoeconomic evaluation process. The industry believes Enhertu can meet the criteria for innovative new drugs and receive preferential treatment. Although Enhertu’s reimbursement passed the Health Insurance Review and Assessment Service (HIRA) Cancer Disease Deliberation Committee’s review after redeliberation in May and set reimbursement standards, it has been unable to pass the Drug Reimbursement Evaluation Committee evaluation ever since. The agenda is said to be stuck in the pharmacoeconomic evaluation stage.
- Policy
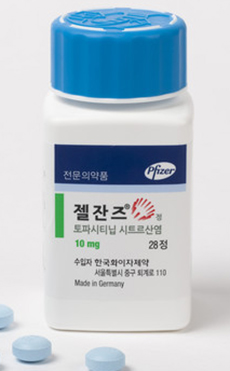
- Only CKD and Jeil have high-dose Xeljanz generics approved
- by Lee, Hye-Kyung Dec 13, 2023 05:38am
- Pfizer Jeil Pharm received approval for its ‘Topazan Tab 10mg,’ a high-dose generic version of the rheumatoid arthritis treatment ‘Xeljanz (tofacitinib citrate).’ With the Ministry of Food and Drug Safety’s approval of Topazan Tab 10mg on November 11 in addition to the Topazan Tab 5mg that was approved on November 27, Jeil Pharm now owns both low- and high-dose generic versions of Xeljanz. Following Boryung, which received approval for ‘Boryung Tofacitinib TAb 5mg (tofacitinib aspartate) as the first Xeljanz generic in August 2020, 58 generics have been approved to date, but Chong Kun Dang is the only company that received approval for both the 5mg and 10mg doses. Reasons include safety concerns and reduced indications associated with the higher dosage formulation, and even the outpatient prescription amount of the original shows this clear difference in demand between the low and high doses. The low dose is approved for five indications including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, polyarticular juvenile idiopathic arthritis (pJIA), and juvenile psoriatic arthritis, whereas the indication for the high dose formulation is limited to ulcerative colitis. In addition, post-marketing surveillance studies of Xeljanz have shown that the drug is associated with an increased risk of blood clots in ulcerative colitis. The MFDS also added a warning to the drug’s label in 2020, cautioning healthcare providers to evaluate the risk of thrombosis in patients with ulcerative colitis and to cautiously use Xeljanz 10mg in patients with risk factors for thrombosis. With persisting safety concerns over the higher dose, according to the market research institution IQIVA, out of the KRW 15.9 billion sales of Xeljanz in 2021, the 5mg dose accounted for about KRW 13 billion, while the 10mg dose accounted for only a mere KRW 2.1 billion. Meanwhile, Xeljanz owns a substance patent that expires in November 2025 and a crystalline patent that expires in November 2027. 20 companies, led by Chong Kun Dang, have tried to overcome it by filing an invalidation trial, and 16 companies including Boryung Pharmaceutical, have tried to avoid the patents by filing a passive scope of patent rights trial. As a result, generic companies succeeded in avoiding Xeljanz’s patent in January 2018, and then won the invalidation trial in November 2019, thereby removing the crystalline patent from the green list in March 2020. As a result, Xeljanz generics that are being granted marketing authorization will be launched after November 2025, when the substance patent expires.
- Policy
- KIDS ‘Oral VEGFR-TKI use may increase risk of AAD’
- by Lee, Hye-Kyung Dec 12, 2023 05:38am
- The Korea Institute of Drug Safety & Risk Management (President: Jeong Wyan Oh, KIDS) announced that the use of Vascular Endothelial Growth Factor Receptor-Tyrosine Kinase Inhibitor (VEGFR-TKI) was associated with an increased risk of aneurysm and artery dissection occurrence. The findings were published in the internationally recognized American Medical Association (AMA) journal, JAMA Network Open (IF=13.8) on November 29. Recently, the U.S. FDA identified an association between VEGF-TKI inhibitors such as sorafenib and pazopanib and the development of aneurysms and arterial dissection through an analysis of the FDA Adverse Event Reporting System (FAERS) data and reflected the results in the indication of identified drugs. Korea’s Ministry of Food and Safety also reflected the association in the indications of affected drugs after conducting a safety information review. The MFDS conducted a study to investigate the risk of aneurysm and arterial dissection associated with the use of VEGFR-TKIs in the Korean population, after deeming that prior epidemiologic studies were lacking in Korea. The study collected information on 127,710 cancer patients aged 40 years or older who were prescribed VEGFR-TKIs or capecitabine based on the National Health Insurance Service’s insurance claims data (2007-2020) and followed them for 1 year after use. Among patients receiving VEGFR-TKIs, the incidence of AAD within 1 year of treatment initiation was 6.0 per 1000 person-years (1,000 subjects observed for 1 year). The incidence of AAD in those receiving capecitabine was 4.1 per 1000 person-years, showing that the risk of AAD occurrence was significantly higher by 1.48 times (95% CI, 1.08-2.02) among patients prescribed VEGFR-TKIs than those receiving capecitabin. The incidence and risk of AAD were significantly higher among patients treated with VEGFR-TKIs than those treated with capecitabine in females (2.08 times), older adults aged 65 years or older (1.42 times), and patients with dyslipidemia (1.58). KIDS President Oh said, “The study holds significance as that it demonstrated the possibility of an association between aneurysm and arterial dissection and the use of VEGFR-TKIs in cancer patients in Korea. This is expected to not only increase the treatment effect but also reduce socioeconomic losses and contribute to building a safer drug use environment."