- LOGIN
- MemberShip
- 2025-12-21 18:20:37
- Policy
- Welireg·Camzyos are approved in Korea
- by Lee, Hye-Kyung May 25, 2023 05:46am
- Welireg, a treatment for von Hippel-Lindau and VHL disease characterized by multiple tumors, and Camzyos, which is used to improve motor function and symptoms in patients with NYHA class II-III obstructive hypertrophic cardiomyopathy, received domestic product approval. It announced on the 24th that it had approved Welireg of MSD Korea and Camzyos of BMS Korea. First of all, Welireg is a drug that inhibits HIF-2α related to cell proliferation, angiogenesis, and tumor growth. It can reduce the risk of surgical excision of the tumor. Hippel-Lindau disease is a rare disease in which incurable multiple tumors occur in the kidney, central nervous system, and pancreas due to mutations in tumor suppressor genes. This drug is used for the treatment of renal cell carcinoma, central nervous system hemangioblastoma, and pancreatic neuroendocrine tumor that does not require immediate surgery in adult patients with von Hippel-Lindau disease, providing new treatment opportunities for patients with these rare diseases. Camzyos is the first treatment for symptomatic obstructive hypertrophic cardiomyopathy in Korea and is expected to provide a new treatment opportunity for patients who have previously used only symptomatic therapy to relieve symptoms. Camzyos has been approved for 4 doses (2.5mg, 5mg, 10mg, 15mg) and relieves excessive contraction of the heart muscle by inhibiting cardiac myosin, and is effective in improving the motor function and symptoms of the patient. The Ministry of Food and Drug Safety said, "We will continue to do our best to expand treatment opportunities for patients with rare and intractable diseases by promptly supplying treatments whose safety and effectiveness have been sufficiently confirmed based on regulatory scientific expertise."
- Policy
- Domestic medical device market share ↑50%
- by Lee, Hye-Kyung May 24, 2023 08:26pm
- The share of domestically produced medical devices exceeded 50%. It is believed that the reason is that the production of medical devices such as in vitro diagnostic devices increased as public health medical products were approved for emergency use after Corona 19. Chae Gyu-han, head of the Medical Device Safety Bureau of the Ministry of Food and Drug Safety, said at a press briefing on the 23rd, “We are analyzing the production status of the medical device industry last year, and the market share of domestic manufactured products exceeded 50%.” It means that we can concentrate on producing essential medical devices for public health when an outbreak occurs and create a system that can stably supply them.” Therefore, the Ministry of Food and Drug Safety has expressed its will to improve the system so that companies with competence do not enter the market due to a lack of licensing experience. Manager Chae said, “The core of Regulatory Reform 2.0, which will be announced soon, is to ensure that the medical device industry develops in line with changes in the policy environment.” " said. Although the domestic medical device industry has developed due to the specificity of COVID-19, it is said that the content of Regulatory Innovation 2.0 will be to prepare an evaluation system to create international-level medical products with prepared technology and experience and to lead the medical device market. While Aimmed's 'Soames' and Welt's 'PillowRx' were recently approved as the 1st and 2nd digital treatment devices (DTx), it also indicated that it would prepare for the development of AI big data-based digital medical products in the future. Manager Chae said, "Deputy Director Oh Yoo-gyeong is also very interested in AI-based medical product development. The Power of the People Rep. Jong-heon Baek and Young-seok Seo of the Democratic Party each proposed the 'Digital Medical Products Act as part of this concern. The bill defines digital medical devices, digital convergence medicines, and digital medical and health support devices as digital medical products prepares an evaluation system and evaluates actual use, introduces an excellent management system certification system, and provides preferential treatment for health insurance benefits. there is. Manager Chae said, "It is expected that the bill will be discussed in the legislative subcommittee, and the management system has been established in line with the era of digital transformation so that digital technology can be applied to medical devices and medicines and used for health care." We will make it legislative,” he said. In line with the enactment of the bill, the Ministry of Food and Drug Safety is also preparing guidelines for the development of digital treatment devices. Manager Chae said, "Even before the legislation, we will prepare guidelines necessary for clinical trials and the development of digital treatment devices." In the field of innovative medical devices and innovative diagnostic devices, he emphasized investment support for selection and concentration. Manager Chae said, “There were concerns about the growth of overall medical devices and the growth of specialized fields, and as a result of conversations with the industry, there were many opinions that selection and concentration were needed.” “I think innovative medical devices and diagnostic devices have competitiveness. We plan to develop areas that can be developed and promote measures such as intensive support.” "Regulatory Innovation 2.0 and the promotion of the Digital Medical Products Act are one of the important projects this year," said Joo Seon-tae, head of the Medical Device Policy Department, who was present at the briefing of the Director of the Medical Device Safety Bureau. did. Seong Hong-mo, head of medical device management, said that she is promoting a project to prepare braille and sign language videos for medical devices to improve information access for the disabled. He said, "The recently revised bill contains information related to information accessibility for the disabled, and it will be a recommendation, not an obligation." He added, "If the sub-law is enacted, it will be conducted by meeting with disabled groups, investigating products that require braille or sign language videos, and recommending them to companies."
- Policy
- Domestic DM combi drugs selected for market competitiveness
- by Lee, Tak-Sun May 23, 2023 05:50am
- LG Chem and Dong-A ST are attracting attention by listing their DPP4i+SGLT2i combination as benefits, lowering the amount added. It is interpreted as being conscious of price competitiveness. According to the industry on the 22nd, Dong-A ST Sugadapa set the upper limit lower than the formula based on addition. This drug is an improved new drug combination, so it was able to receive a drug price that combines 59.5% of the individual ingredients. If there is no addition, you will receive the sum of 53.5% of the individual ingredients. For example, 434 won, 59.5% of 730 won for Evogliptin 5mg and 393 won, 59.5% of 734 won for Dapagliflozin 10mg, was the amount Sugadapa could receive. Dong-A ST decided to list it at 799 won, lower than the formula. As a result, it became the product with the lowest upper limit than the SGTL2!+DPP4i complex, which was released first last month. For the same reason, LG Chem's Zemidapa was also listed lower than the added amount. Zemidapa was able to receive the sum of 68% of the individual ingredients as an innovative new drug complex. The sum of 509 won, or 68% of 749 won for Gemigliptin 50mg and 499 won, or 68% of 734 won for Dapagliflozin 10mg, was able to receive 1,008 won. Zemidapa was also listed at a lower price of 940 won. However, even though the price of Zemidapa has been lowered, the upper limit is higher than that of competing drugs. After one year, the price of the two drugs will drop to the sum of 53.55% of the individual ingredients. Sugadapa is 784 won, and Zemidapa is 794 won. In any case, this is because a lower addition than the existing formula was applied. The low pricing of the two pharmaceutical companies is attracting attention in that they usually try to get a higher upper limit. This can be interpreted as an intention to induce the market to settle down by lowering the price, as metformin + SGLT2i + DPP4i has been covered by reimbursement since April, and the SGLT2i + DPP4i combination was released for the first time in May. Moreover, it seems to be aware of the competition as not only the two domestic products but also four other complexes were released.
- Policy
- Myelofibrosis tx BMS Inrebic to be covered from June
- by Lee, Tak-Sun May 22, 2023 05:42am
- BMS Korea's myelofibrosis drug Inrebic will be covered from June. This drug is expected as a second-line treatment option for myelofibrosis patients, of which there are about 1,700 in Korea. According to the industry on the 19th, Inrebic will be listed as a salary at 39,520 won per capsule for the maximum amount from June 1st. Inrevic Capsule is indicated for the treatment of splenomegaly or symptoms associated with the following diseases in adult patients previously treated with Ruxolitinib (Brand Name: Jakavi). The following diseases are ▲primary myelofibrosis, ▲myelofibrosis after polycythemia vera, ▲and myelofibrosis after essential thrombocythemia. In February, it passed the HIRA Cancer Disease Review Committee and the Pharmaceutical Reimbursement Evaluation Committee, and after negotiations with the NHIS, it was listed as reimbursement in June. Inrebic is the first medicine to treat myelofibrosis in 10 years. Myelofibrosis is a rare hematological cancer in which normal hematopoietic function is impaired along with the excessive fibrotic proliferation of the bone marrow. Patients experience symptoms that affect their quality of life, including an enlarged spleen, fatigue, itchiness, weight loss, night sweats, fever, and bone pain. Until now, patients with myelofibrosis had no alternative when treatment with the JAK inhibitor Jakavi failed, but with the release of Inrebic, a second treatment option was created. Jakavi is a blockbuster drug with global sales of $388 million last year.
- Policy
- Vemlidy’s price 4.7% ↓...Vemliver’s voluntarily 12.6% ↓
- by Kim, Jung-Ju May 22, 2023 05:42am
- Gilead’s adult chronic hepatitis B treatment, ‘Vemlidi Tab (tenofovir ala fenamide) will be subject to Price-Volume Agreement price cuts and be sold at a 4.7% lower price starting next month. Daewoong Pharmaceutical opted to reduce the price of its latecomer Vemliver Tab by 12.6%. According to industry sources on the 21st, the Ministry of Health and Welfare (MOHW) ‘Amendment to the drug reimbursement list and reimbursement ceiling price table’ contains the abovementioned changes that will become effective as of the 1st of next month. ◆Price cut for PVA drugs = The PVA price cut will be applied to two products next month, and the two drugs underwent PVA negotiations with the National Health Insurance Service, one as a Price-volume agreement type ‘A’ and the other as a Price-volume agreement type ‘B’ drug. First, the drug that completed negotiations as a PVA Type A drug was Abbvie’s Rinvoq (upadacitinib), and its price will be reduced by 0.9%. Among new drugs listed as negotiable after the introduction of the drug price negotiation system, the government applies PVA A type to drugs whose claims amount in the same product category exceeds the expected claims amount by over 30%. By same product category, drugs that have the same company name, route of administration, ingredient, and formulation are classified as drugs of the same product category.’ The drug subject to ‘Type B’ price reduction was Gilead’s Vemlidy Tab, and its price was cut by 4.7%. The government applies PVA Type B and reduces the price of drugs ▲whose price ceiling had been already adjusted according to Type A, ▲ whose claims amount increased by over 60% from the previous year without undergoing Type A negotiations, or 4 years after receiving Type A negotiations, or ▲ has increased by over 10% but the increased amount exceeds KRW 5 billion and does not fall under PVA Type A. ◆Products that submitted voluntary price cuts= Five products in total decided to make voluntary price cuts. When a pharmaceutical company submits a request to lower its drug price to an amount lower than the ceiling price set for the product, the government adjusts the insurance drug price of the product to the requested amount. One voluntary price cut for a latecomer drug was made in line with the PVA price cut of Vemlidy. Daewoong Pharmaceutical opted to make a double-digit reduction in the price of its latecomer Vemliver Tab, by 12.6%. BMS Korea decided to make a price cut in the 1% range for each of its dosage strengths of Baraclude (entecavir). Janssen Korea’ made a voluntary price cut of 0.5% each for its Tremfya Prefilled Syringe (guselkumab) and Tremfya Autoinjection Inj.
- Policy
- Revival of omega-3 fatty acids...4g high-dose recommended
- by Choi, sun May 18, 2023 05:45am
- The Korean Society of Lipid and Atherosclerosis (KSoLA) disclosed the full version of its 5th edition of the Korean Guidelines for the Management of Dyslipidemia, in which the use of omega 3 was subdivided into the use of 'high dose and refined ingredients'. Although there has been controversy over its efficacy, the new guideline puts weight on the fact that the benefits of its use still outweigh its non-use. Annual Scientific Conference of the KSC with Affiliated Cardiac Societies On the 22nd, the Annual Scientific Conference of the KSC with Affiliated Cardiac Societies that was organized by 8 academic societies including the Korean Society of Cardiology, the Korean Heart Rhythm Society, the Korean Society of Heart Failure, and KSoLA, announced the ‘2023 KSoLA Guideline Update,’ ‘Evidence of Guidelines,’ and ‘Limitations in the Evidence’ were announced. A simplified version of the revised guidelines was announced last year, but this year, the full version was released to support its details and rationale. The new changes made in the 5th version were ▲the Method of diagnosis and standards, ▲Treatment standards. First, in the new diagnosis method and standard, the KSoLA showed the result that the non-fasting triglycerides level showed a high correlation with the risk of cardiovascular disease. In the standards, patients with coronary artery disease were recommended to lower their LDL cholesterol target level from less than 70 mg/dL to less than 55 mg/dL and lower it by more than 50% from baseline. In the case of patients with diabetes, the target goal is less than 70 mg/dL for patients with a disease duration of 10 years or more, one or more additional risk factors, or target organ damage, depending on the risk level. In addition, diabetic patients with 3 or more target organ damage or major cardiovascular disease risk factors can selectively consider reducing LDL cholesterol to less than 55 mg/dL. Furthermore, as exercise therapy, the recommendation grade for the use of wearables for fitness was newly presented as IIa, and additionally methods for using wearable equipment and a strategy for promoting physical activity were suggested. In the full version, society took a somewhat reserved position on the use of Omega 3, which was plagued by controversy over its cardiovascular protective effect. Professor Ye-Seul Yang (Endoctrioinology, SNUH), who presented on the 'New Changes on the KSoLA Guidelines,’ said, “The 5th edition of the revised guidelines segmented and reinforced the treatment criteria and treatment targets for dyslipidemia. The new guideline recommended different treatment standards by subdividing diabetes by risk group." Yang said, “Individualized guidelines were set according to specific groups such as those with stroke, chronic kidney disease, the elderly, adolescents, and familial hypercholesterolemia. In terms of drug therapy, the recommended grade was raised to emphasize that statins are a first-line treatment drug, and introduced icosapent ethyl(IPE) and the role of fibrate and omega-3 fatty acids in managing triglyceride.” As for whether omega 3 actually shows a protective effect against cardiovascular disease, large-scale studies have come to different conclusions. The most recent study that proved its efficacy observed an effect when only the IPE component was isolated from the omega-3 and used in high dosages. The KSoLA newly included the use of IPE in the recommendation for patients with atherosclerotic cardiovascular disease or diabetes who still have hypertriglyceridemia even after LDL cholesterol is controlled below the target level with statins. In the treatment of hypertriglyceridemia, "Use of fibric acid derivatives or omega-3 fatty acids that mainly lower triglycerides first are recommended as a priority (IIa, A), and even after achieving LDL cholesterol below the target level through therapeutic lifestyle improvement and statin drug treatment, if the triglyceride is 200 mg/dL or higher or the non-HDL cholesterol level is higher than the target value, drug treatments to lower the triglyceride can be considered (IIa, B)." KSoLA said, "In the recent REDUCE-IT study, 4g of IPE was administered per day in patients at high risk of atherosclerotic cardiovascular disease or diabetes, which reduced the incidence of cardiovascular disease by 26% compared to placebo. If hypertriglyceridemia persists in high-risk patients at a 200 mg/dL or higher level even after lifestyle improvement and statin administration, additional IPE (4g per day) can be administered to prevent cardiovascular disease (IIb, B)." However, KSoLA added a provision on account of the controversy in place. KSoLA added, “The effect of omega-3 fatty acids on reducing the risk of cardiovascular disease is still in controversy because of the varying results shown depending on formulation or dosage. In the recent REDUCE-IT study that was conducted in patients at high risk of cardiovascular disease whose triglyceride levels were higher than 135-499 mg/dL, the use of IPE, a high-purity EPA, twice a day significantly reduced death from cardiovascular disease and the occurrence of ischemic disease." “In the European practice guidelines that were revised in 2019, control of triglyceride using IPE was recommended for patients at high risk of cardiovascular disease, however, IPE is not being sold in the market yet. Also, in another recent study, the STRENGTH study, the combination of EPA and DHA did not show any benefit in cardiovascular disease, but rather increased the risk of atrial fibrillation compared to the control group." Also, the guidelines emphasized the need to use a high-dose high-purity ingredient for the combined use of statin and Omega 3. KSoLA said, “Combination therapy can be used to reduce LDL cholesterol and triglyceride at the same time. "The combination of 4 g of Omega 3 per day with simvastatin significantly decreased triglyceride and slightly increased HDL cholesterol."
- Policy
- Initial appvl rate of drugs subject to prior review varies
- by Lee, Tak-Sun May 18, 2023 05:45am
- As a result of analyzing the approval rate of prior authorization drugs over the past 10 years, the approval rate varied greatly according to the type of drug. However, unlike during the initial review, the review for maintenance therapy showed a high approval rate of 90%. Yong-Kyun Won, Professor of Radiation Oncology at Soonchunhyang University Cheonan Hospital, announced so through a retrospective record analysis study on the prior authorization drugs over the past 10 years (2021-2022).' The study was presented at the 22nd Annual Conference of the Korean Society of Insurance Medicine which was held on the 14th. The prior authorization system was implemented in 2012 to establish clear standards for the use of high-priced drugs and to prevent drug abuse. Soliris, Spinraza, Ultomiris, Strensiq, and Zolgensma, which are rare disease drugs and ultra-high-priced drugs that cost more than KRW 300 million won per year, receive health insurance reimbursement through the system. Crysvita was recently added as a drug that requires prior authorization. According to the study, drugs that were expensive but are essential for the treatment of rare diseases have been able to receive reimbursement through the system, and this pre-deliberation system has been successful, such as in managing the quality of treatment through patient monitoring (maintenance therapy), etc. However, the approval rate was different for each drug. In particular, the prior authorization approval rate for initial administrations ranged from 20% to 100% by product or indication. In comparison, the review approval rate for maintenance therapy exceeded 90%. The varying initial approval rate of prior authorization drugs (retrospective record analysis study on prior authorization drugs (2012~2022)) For example, in the case of patients who seek to use Soliris for aHUS, the initial approval rate was only 21.6%. On the other hand, drugs such as Ultomiris (77.8%) and Strensiq (100%) showed high approval rates. On the other hand, the number of acceptations of objections on the disapproval was low. Only 1 out of 17 objections in 2022 were accepted, and therefore the analysis was that it was a difficult environment for disapproved drugs to receive deliberations again. Professor Won expressed concerns about how the low approval rate may limit access to reimbursement. In addition, for drugs in need of urgent deliberations due to the child's age or disease type, Won explained that the system where institutions need to wait for announcements until the end of the month to see why their application was disapproved and what needs to be supplemented, should be improved as well. Professor Won said, “The prior authorization system is settling as an essential system in securing access to treatment for high-priced drugs that are being continuously introduced to the field. Doctors may feel it is difficult to use a drug if the approval rate is too low. Therefore, it is necessary to review whether the reimbursement standards are too strict and whether it needs revisions.”
- Policy
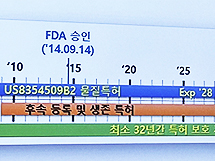
- Patent protection for 42 yrs for Humira/32 yrs for Keytruda
- by Lee, Hye-Kyung May 16, 2023 09:07pm
- It was analyzed that Humira maintained its patent protection period for at least 42 years and Keytruda for at least 32 years after filing for material patents with the Evergreening patent strategy. Evergreening refers to extending the term of a patent or extending the term of a patent for more than 20 years in the case of a patent to obtain more exclusive rights during the patent protection period. The types of evergreening strategies are representative of salt compounds, solvates, crystalline forms, optical isomers, dosage forms and pharmacokinetic data, manufacturing methods, and uses. Kim Tae-Kwon, responsible for the Korea Institute of Patent Technology Advancement, held on the last day of the 'Bio Korea 2023' event held on the 12th, 'Strategies for responding to original drugs and late-comer drugs according to the expiration of blockbuster drug patents'. Introducing the patent strategy. Kim Tae-kwon, head of the Korea Institute of Patent Technology Promotion The reason Humira and Keytruda are cited as examples is that the two medicines, excluding vaccines, respectively, ranked first and second in global sales in 2021, with sales of Humira at $20.694 billion and Keytruda at $17.186 billion that year. Humira applied for a substance patent in the United States in 1996, was registered as a patent in 2000, and received FDA approval in 2002. Since then, it has received FDA approval for additional indications such as psoriatic arthritis, ankylosing spondylitis, and Crohn's disease, and the substance patent period expired in 2016. So far, Humira has confirmed 746 patents based on 79 original patent applications. By type, 23 family groups (29.11%) for medicinal use and manufacturing method were distributed the most, followed by other 9 (11.39%), 8 diagnoses (10.13%), 7 formulations (8.66%), and 4 compositions. (5.56%), and 2 material improvements (2.53%). Responsibility Kim said, “Before the substance patent of Humira expired, we continuously extended the patent period with patents for medicinal use, formulation and composition patents, manufacturing method patents, diagnostic patents, automatic administration device patents, and pharmaceutical improvement patents.” It maintained its monopoly." In other words, Humira used an ever-greening patent strategy, such as forming a relatively superior patent barrier in the field of pharmaceutical use and manufacturing methods and forming a patent barrier in the pharmaceutical field as well. As a result, Humira was able to have a protection period of at least 42 years after applying for a substance patent and 35 years after FDA approval. After applying for a material patent in 2008, Keytruda registered a patent in 2013 and received FDA approval in 2014. Based on 67 original applications for patents, a total of 345 patents were confirmed. Looking at the distribution by type, 48 (71.64%) of the medicinal uses showed an overwhelming distribution. Next, 7 drugs (10.45%), 4 double antibodies (5.97%), 3 substances (4.48%), 3 diagnoses (4.48%), and 2 others (2.99%) are shown. Kim said, "There was also a case where the dosage and usage were changed as a barrier patent for the evergreening strategy of Keytruda products." "There was also data that it was to block the development of biosimilars as the patent term expires in 2028." and interpreted. In the case of Keytruda, it used the evergreening patent strategy, and it was analyzed that it had a protection period of at least 32 years after the substance patent and about 26 years after FDA approval. Kim explained, "As a result of the analysis of patent applications by type of biopharmaceutical, material patents must be filed, and based on material patents, applications are filed in the order of pharmaceutical use, composition, formulation, manufacturing method, diagnosis, and material improvement patents." As a result of comparing the evergreening strategy of biopharmaceuticals and synthetic drugs, there are patents for use, manufacturing methods, and formulations after substance patents, but synthetic drugs have many improved patents such as crystal forms, optical isomers, polymorphs, and intermediate patents for chemical formulas. However, as for biopharmaceuticals, there were patents for improving antibody or protein drugs. In addition, substance patents are important, but in the case of manufacturing method patents, it was noted that the distribution of biopharmaceuticals was high and the distribution of synthetic drugs was low. Kim said, "One thing to look carefully at is the expiry of the duration of a biopharmaceutical, but we need to look at changes in the patent for formulation, usage, and dosage in the use patent."
- Policy
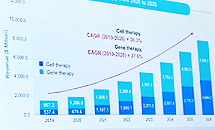
- Biopharmaceutical CDMO annual average of ↑31%
- by Lee, Hye-Kyung May 15, 2023 05:41am
- Kwon Soon-jae, managing director of ENCell, is giving a presentation on the current status of the CDMO market at Bio Korea 2023.Globally, the CDMO market for biopharmaceuticals is growing at an average annual rate of 31%. If this trend continues, the size of the Cell&Gene Therapy CDMO market is expected to reach 10 billion dollars in 2026. Kwon Soon-jae, managing director of ENCell, announced this at the 'CDMO Partnership for Acceleration of Biopharmaceutical Development session held at the 'Bio Korea 2023' event held on the 10th. Director Kwon explained, "Cell & Gene Therapy is expected to grow 5.5 times in 2026 compared to 2019, and gene therapy is expected to grow 8.7 times." He explained, "If you look at the high CAGR from 2019 to 2026, it will account for 36.3% and 27.6%, respectively." The demand for CMOs and CDMOs has increased due to the COVID-19 pandemic, and Director Kwon said, "Small companies use CMOs and CDMOs to reduce costs and time, while large companies use CMOs and CDMOs to reduce marketing and R&D costs." It plays a part," he said. However, in the case of domestic CGT treatment, the manufacturing technology is complicated and the number of platforms is not large, so it was inevitable to create a GMP facility with an 'in-house' concept rather than CDMO service, and have many in-house processes and services. Director Kwon said, "However, as the requirements of the Ministry of Food and Drug Safety become stricter, infrastructure, raw materials, facility costs, labor costs rise, and technology becomes more complex, outsourcing instead of in-house is becoming a trend." Looking at the domestic CGT CDMO market, Lonza, Samsung Bio, SK, CJ, Lotte, and Medipost have announced their entry into the CMO/CDMO business following Thermoficer in 2017. Director Kwon said, "Most of the 30 CGT companies in Korea are major companies, and 80% of them are trying to develop AAC, adenovirus, CAR-T, etc., and only 30% of them are investing more than 2 million dollars." "If you look at the CGT market alone, it's still the first step, the introductory stage," he said. Director Kwon said that the present, when the first step of CDMO in the CGT market was taken, is an important point in determining the future. Director Kwon said, "More than 100 companies worldwide have entered the CDMO cell gene therapy market, and price, location, and regulations are challenges to be resolved." It looks like I'll have to give it a try," he said.
- Policy
- Youkyung Oh was appointed as the first chairman of APFRAS
- by Lee, Hye-Kyung May 12, 2023 05:44am
- On the 10th and 11th, the Ministry of Food and Drug Safety (Minister Oh Yookyung) held the 1st Asia-Pacific Food Regulatory Authority Heads Consultative Meeting (APFRAS 2023), where 7 countries came together to harmonize global food regulations in the Asia-Pacific region and strengthen cooperation. said to have collected. APFRAS (Asia-Pacific Food Regulatory Authority Summit) is participated by New Zealand, Vietnam, Singapore, China, Philippines, Korea, and Australia. On the 11th, Korea was elected as the first chair of APFRAS at a meeting of heads of food regulatory agencies from seven countries, and Minister Oh You-keong of the Ministry of Food and Drug Safety was appointed as chair. As the chair, Korea established a secretariat, operated a working group, and communicated among member countries for three years. do The member countries adopted the Operational Regulations (TOR) following the establishment of APFRAS, and also resolved implementation tasks for the operation of the working group and achievement of strategic goals. In the future, the APFRAS working group will analyze the food regulatory environment in the Asia-Pacific region and discuss in-depth discussions on the digitalization of food safety management and carbon neutrality in the food sector. In addition, for food safety, we agreed to rapidly analyze new global issues and respond cooperatively to changes in the international food environment, and urge the strengthening of the cooperative system to create a safe food distribution environment in the Asia-Pacific region and solve common tasks. The Declaration was adopted and signed by all seven member states. It was agreed to hold the APFRAS meeting once a year to continue the close cooperation system among member countries, and the second APFRAS meeting in 2024 is scheduled to be held in Seoul, Korea, which was elected as the chair country. Minister of Ministry of Food and Drug Safety Oh Youkyung discussed with Lim Kok Thai, head of the Food and Drug Administration of Singapore, to conclude a food safety cooperation agreement (MOU) to draw common interests between the two countries, such as standards for new food raw materials, and to strengthen cooperation between the two organizations. Director Oh said, "With the launch of APFRAS, the world's first head of a food regulatory agency gathered in one place to discuss various issues related to global food safety and achieve meaningful results by agreeing on strengthening capabilities among regulatory agencies." Director Oh said, "As I was elected as the APFRAS chairman, I will do my best so that Korea can play a leading role in quickly identifying new food safety issues and changes and raising the level of food safety in member countries. I will try,” he said. The Ministry of Food and Drug Safety will continue to lead international cooperation and regulatory harmonization for food safety and continue discussions to resolve non-tariff barriers. .