- LOGIN
- MemberShip
- 2025-12-17 20:28:08
- Company
- Novo Nordisk, Kakao Health sign digital healthcare MOU
- by Cha, Jihyun Sep 04, 2025 06:09am
- Kakao Healthcare (CEO Hee Hwang) announced on the 3rd that it has signed a memorandum of understanding with the multinational pharmaceutical company, Novo Nordisk Korea (General Manager Kasper Roseeuw Poulsen) to build a digital healthcare ecosystem for obesity and diabetes patients. The signing ceremony, held on September 2 at Novo Nordisk Korea’s headquarters in Songpa-gu, Seoul, was attended by Kakao Healthcare CEO Hee Hwang and Novo Nordisk Korea CEO Kasper Rosseeu Poulsen. At the event, the two companies agreed to collaborate by utilizing various digital technologies to improve the treatment journey of obesity and diabetes patients, ultimately creating a sustainable treatment environment that can deliver better outcomes. This is the second collaboration between the two companies, following their first partnership in 2023. Under the agreement, they linked Kakao Healthcare’s AI-based health management app ‘Pasta’ with Novo Nordisk’s insulin FlexTouch pen and its smart cap ‘Mallya,’ providing a medication management solution for diabetes patients. The new partnership is significant in that it expands the scope of collaboration from diabetes to obesity, creating a comprehensive alliance. The companies plan to address the unmet medical needs of Korea’s rapidly growing obesity and diabetes populations by offering patient-centered digital solutions. Specifically, in the field of obesity, the partnership will focus on ▲developing a personalized digital support program, ▲providing solutions to improve treatment effectiveness and quality of life. and In particular, the partnership will integrate Novo Nordisk’s patient support program Novo Fit Care, which is offered to patients prescribed its obesity treatments, into the Pasta app. Through this, patients will be able to manage weight and other long-term health aspects in a comprehensive way. In the field of diabetes, the companies will jointly develop digital solutions aimed at improving disease awareness. Kasper Roseeuw Poulsen, General Manager of Novo Nordisk Korea, said, "Obesity and diabetes are chronic conditions which, if left untreated, can lead to serious comorbidities and impose a tremendous burden on individuals and society. With Novo Nordisk’s more than 100 years of dedication to diabetes and obesity, and Kakao Healthcare’s leadership in Korea’s digital healthcare sector, the partnership will further accelerate integrated innovation for patient support in Korea." Hee Hwang, CEO of Kakao Healthcare, said, "Kakao Healthcare has leveraged AI, big data, and other technologies to bring positive changes to patients’ health journeys. By combining this experience and technology with Novo Nordisk, a global leader in obesity and diabetes care, we aim to play a major role in expanding patient-centered digital healthcare on a global scale."
- Company
- Moderna’s latest variant-targeted COVID-19 vaccine approved
- by Whang, byung-woo Sep 03, 2025 06:08am
- Moderna Korea announced on the 1st that its LP.8.1 variant-targeted COVID-19 vaccine, ‘Spikevax LP Inj’, has been approved by the Ministry of Food and Drug Safety (MFDS). Spikevax LP has been confirmed to induce broad cross-immune responses against currently circulating variants, including the LP.8.1 strain, and it is authorized for use in adolescents aged 12 and above as well as adults. Moderna plans to supply the newly approved vaccine in time for the government’s 2025–2026 seasonal immunization program, which begins in October. The company noted that its COVID-19 vaccines have demonstrated strong immune effect and safety in large-scale Phase III clinical trials and extensive real-world evidence (RWE). A key feature is that elderly individuals aged 65 and above showed immune responses comparable to those in younger adults. Also, regardless of which vaccine type had been administered previously, the Moderna vaccine showed high immunogenicity when used as a subsequent dose. A domestic study conducted by the Korea Disease Control and Prevention Agency (KDCA) also confirmed that Moderna’s vaccine recorded the lowest breakthrough infection rate among the vaccines used in the early stages of the pandemic. According to the National Immunization Program guidelines, Spikevax LP will be provided free of charge to high-risk groups, including seniors aged 65 and over and residents of long-term care facilities. The Korean Society of Infectious Diseases has stressed that immunity acquired through infection or vaccination wanes over time, and with the emergence of new variants, periodic updated COVID-19 vaccination for high-risk groups is recommended. The LP.8.1 series vaccines supplied this season have already been recommended for use by the World Health Organization (WHO), the European Medicines Agency (EMA), and the U.S. Food and Drug Administration (FDA). Based on recommendations from its Vaccination Expert Committee, the KDCA decided to adopt the LP.8.1 vaccine, which demonstrated stronger neutralizing antibody responses compared to last season’s JN.1-based vaccines. Moderna is the only company manufacturing mRNA COVID-19 vaccines in Korea, through its partnership with Samsung Biologics, and continues to ensure a stable vaccine supply via ongoing cooperation with Boryung Biopharma. Sang Pyo Kim, General Manager of Moderna Korea, said, “COVID-19 remains a threat to high-risk groups, with hospitalizations on the rise for 7 consecutive weeks. Moderna is committed to delivering updated COVID-19 vaccines that address the latest variants in a timely manner, ensuring that people can be vaccinated safely.”
- Company
- 'Prevenar 20' now available at general hospitals
- by Eo, Yun-Ho Sep 02, 2025 06:11am
- Product photo of Prevenar 20 PFS The pneumococcal conjugate vaccine 'Prevenar 20,' which will soon be included in the National Immunization Program (NIP), is becoming available for prescription at general hospitals. According to industry sources, Pfizer Korea's Prevenar 20 has passed the drug committees (DC) of tertiary general hospitals, including Samsung Medical Center, Seoul National University Hospital, Asan Medical Center in Seoul, and Sinchon Severance Hospital, and medical institutes, including Kangnam Sacred Heart Hospital, Kyung Hee University Hospital at Gangdong, Pusan National University Hospital, Seoul National University Bundang Hospital, Ajou University Hospital, Pusan National University Yangsan Hospital, and Chungnam National University Hospital. Several of these hospitals, including Seoul National University Hospital, have approved codes for adult patients only. However, as Prevenar 20 will be included in the NIP effective October, more hospitals are expected to have pediatric vaccines. Prevenar 20 is a pneumococcal conjugate vaccine that was approved by the Ministry of Food and Drug Safety (MFDS) on October 31, 2024. Compared to the 13-valent vaccine, Prevenar 20 has added seven additional pneumococcal serotypes. Among domestically approved pneumococcal conjugate vaccines, it contains the most serotypes. In addition to existing serotype components in the 13-valent vaccine, Prevenar 20 contains seven additional serotypes (serotypes 1, 3, 4, 5, 6A, 6B, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, 33F). It can be used to prevent invasive diseases and pneumonia in all ages, including infants aged six weeks and above. Pneumococcus is a major bacterial pathogen that causes various diseases in infants and young children, including otitis media, pneumonia, and meningitis. Vaccination is crucial, especially as it can cause life-threatening invasive pneumococcal disease (IPD) in immunocompromised children. Despite South Korea's NIP supporting the 23-valent pneumococcal polysaccharide vaccine (PPSV23) for adults aged 65 and older, this age group has the highest incidence of invasive pneumococcal disease (IPD). From September 2014 to mid-November 2023, a total of 3,734 cases of IPD were reported. The incidence rate for adults aged 65 and over was 32.1 cases per 100,000 people, accounting for 54.8% of all cases. This rate is the highest among all age groups under 65 years old. The inclusion of Prevenar 20 in the NIP was decided after a comprehensive review by the Korea Expert Committee on Immunization Practices (KECIP) on the vaccine's safety, immunogenicity, and cost-effectiveness. Meanwhile, Korea Vaccine is responsible for the domestic promotion of pediatric Prevenar 20, while Chong Kun Dang handles the promotion of the adult vaccine.
- Company
- Patent suits active to release Rinvoq generics
- by Kim, Jin-Gu Sep 02, 2025 06:10am
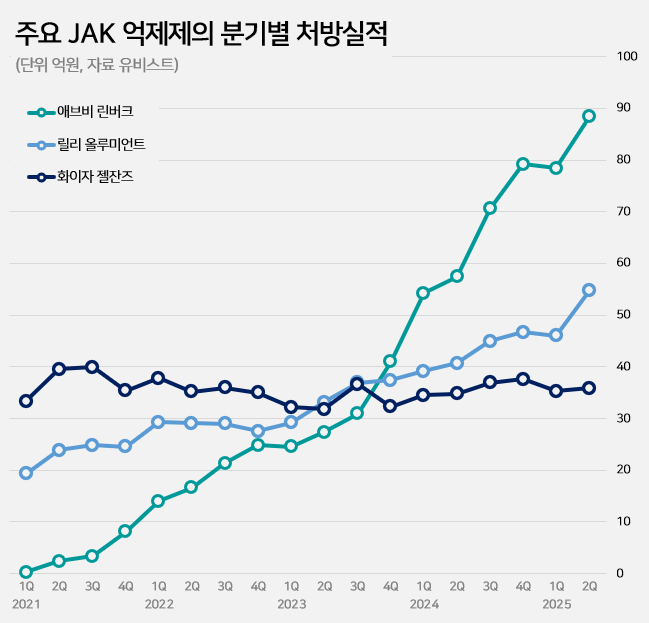
- Pic of Rinvoq The race to launch generics of AbbVie’s Rinvoq (upadacitinib), an oral treatment for autoimmune diseases, is showing signs of intensifying patent disputes. With the JAK inhibitor market expanding rapidly and Rinvoq solidifying its dominance, multiple companies are expected to mount patent challenges. According to industry sources on the 1st, Daewoong Pharmaceutical recently filed a passive scope confirmation trial against AbbVie, challenging the crystalline form patent of Rinvoq. Currently, two patents related to Rinvoq are registered: a substance patent expiring in May 2032 and a crystalline form patent expiring in October 2036. Daewoong's strategy is to first circumvent the crystalline form patent and then launch a generic version early, timed for the expiration of the substance patent. Chong Kun Dang opened the door to this dispute by challenging Rinvoq’s patents before Daewoong Pharmaceutical. Chong Kun Dang filed a related trial request on the 19th of last month. The pharmaceutical industry expects more companies to challenge Rinvoq’s patents from here on. One of the key requirements for obtaining first generic exclusivity is filing the initial invalidation trial. However, if another company files the same trial within 14 business days of the first filing date, it is also deemed to meet this requirement. This is why additional trial filings are expected in the coming weeks as companies move to secure first generic exclusivity for Rinvoq. According to UBIST, a pharmaceutical market research institution, outpatient prescriptions of JAK inhibitors in the first half of this year reached KRW 38.6 billion, up 40% from the KRW 27.5 billion in the same period last year. JAK inhibitors are experiencing rapid growth in the autoimmune disease treatment market, driven by the convenience of being oral formulations. The JAK inhibitor market, which was KRW 18.7 billion in 2020, grew by 36% to KRW 25.5 billion the following year. It continued to grow significantly each year: KRW 35.5 billion in 2022, KRW 40 billion in 2023, and KRW 62.2 billion in 2024. The expanded reimbursement granted for key products drove this market growth. Growth accelerated further last October when switching between JAK inhibitors for rheumatoid arthritis was allowed reimbursement. Major JAKi Prescriptions (AbbVie During this market expansion, Rinvoq has tightened its grip as the dominant player. In the first half of this year, Rinvoq recorded KRW 16.7 billion in prescriptions, a 49% increase from KRW 11.2 billion in the same period last year. Its growth has outpaced competitors such as Xeljanz (tofacitinib) and Olumiant (baricitinib). Having claimed the number one position in Q4 2023, Rinvoq has since expanded its market share, which now stands at 43% in the first half of this year. Rinvoq is a JAK inhibitor used for autoimmune diseases such as rheumatoid arthritis and atopic dermatitis. Its mechanism involves inhibiting inflammatory cytokines, thereby suppressing inflammation, pain, and cell activation.
- Company
- China's presence rises amid early-stage ADC candidate deals
- by Son, Hyung Min Sep 02, 2025 06:10am
- With competition in the antibody-drug conjugate (ADC) market intensifying, global deal trends are undergoing a clear shift. Transactions are now more active in the preclinical and early clinical stages, rather than in late-stage candidates, with collaborations between global pharma and Chinese companies standing out. Since the success of Enhertu, topoisomerase I (Topo I) payloads have effectively become the dominant approach. On the 29th, AimedBio and Samsung Medical Center held the 3rd ADC Conference at the hospital’s main auditorium, where AimedBio Chairman Nam-Gu Her highlighted recent changes in the ADC industry landscape. ADC therapies are novel anticancer drugs that link an antibody that is designed to bind a specific antigen on the surface of cancer cells with a cytotoxic payload through a linker. This design allows the drug to selectively target cancer cells, enhancing efficacy while minimizing adverse effects. According to Her, the ADC market has seen explosive growth since the launch of Enhertu in 2019. Over the past 5 years, deals worth USD 390 billion (KRW 540 trillion) have been completed, with more than 300 clinical trials initiated. The ADC Deal Boom and Trials Enhertu is a next-generation ADC composed of a monoclonal antibody that has the same structure as trastuzumab, which binds to receptors overexpressed on cancer cells and a highly potent Topo I inhibitor payload, linked via a tumor-selective cleavable linker. In a head-to-head study, Enhertu nearly doubled progression-free survival (PFS) compared to Kadcyla, which uses the same trastuzumab backbone but with a microtubule inhibitor payload. “Most companies are now developing ADCs with Topo I inhibitor payloads. In addition to Enhertu, Datroway, and Trodelvy are such examples,” said Her. In the past 5 years alone, 59 deals have been struck involving Topo I payloads, far outpacing tubulin inhibitors (28), degraders (11), immunomodulators (8), and DNA-damaging agents (5). Heo also noted that licensing dynamics have shifted. Historically, big deals centered on acquiring late-stage or commercial products. Today, however, activity has increased around preclinical candidates. High-profile examples made in the past include AstraZeneca’s partnerships on Enhertu and Datroway, AbbVie’s acquisition of Elahere, and Pfizer’s acquisition of Adcetris. However, nowadays, the same big pharma are actively scouting promising preclinical ADC assets. In the ADC payload development landscape, the use of topoisomerase I inhibitors has surged explosively. “The traditional deal trend was to acquire products rather than platforms. While deal sizes have decreased recently, interest in ADCs has not waned,” Her explained. “With many leading candidates already acquired or commercialized, transactions are now focusing on early-stage pipelines.” Chinese pharmaceutical companies are playing an increasingly prominent role. Technology exports from China surged from 55 cases in 2015 to over 300 in 2021, and recorded 213 last year. For instance, in 2022, MSD secured global rights to the TROP2 ADC sacituzumab tirumotecan from Kelun-Biotech for USD 1.41 billion. Last year, GSK obtained global rights to a B7-H3-targeting ADC from Hansoh Pharma. “The mainstream deal trend nowadays is acquiring on preclinical candidates through small-scale transactions. Global big pharma’s interest in Chinese firms is growing rapidly, reflecting China’s strengthened competitiveness in the ADC development scene.”
- Company
- How will fate fare for Roche’s two anticancer drugs?
- by Eo, Yun-Ho Sep 01, 2025 06:05am
- The industry’s eyes are on whether Roche's two anti-cancer drugs, which have faced repeated failures, will succeed in securing reimbursement listing this time. According to industry sources, Roche Korea's treatment for refractory diffuse large B-cell lymphoma(DLBCL), ‘Polivy (polatuzumab vedotin)’ and the PD-L1 inhibitor-based immunotherapy ‘Tecentriq (atezolizumab)’ passed the Health Insurance Review and Assessment Service's Cancer Disease Deliberation Committee last month and are currently under discussion for submission to the Drug Reimbursement Evaluation Committee. Both drugs failed to clear the Cancer Disease Deliberation Committee hurdle twice before finally passing on their third attempt. Therefore, it remains to be seen whether Roche, a pharmaceutical company specializing in anticancer drugs that has particularly struggled with Cancer Disease Deliberation Committee approvals since last year, can now achieve progress simultaneously in solid tumors and hematologic malignancies. Polivy originally aimed for reimbursement in 2021 for its first indication - third-line therapy in combination with BR therapy (bendamustine and rituximab), but failed to clear the CDDC hurdle. In the first half of 2023, Roche submitted a reimbursement application for Polivy’s use as first-line therapy in combination with R-CHP therapy (rituximab + cyclophosphamide, doxorubicin, prednisone). However, this application also faced rejection by the CDDC in February of last year. However, there is still hope. Polivy added the 60.9-month follow-up analysis results from the POLARIX study, which evaluated the efficacy of the Pola-R-CHP combination therapy as first-line treatment for DLBCL last year. In the case of Tecentriq, the drug’s reimbursement was first submitted to the Health Insurance Review and Assessment Service (HIRA) for review in May 2023, but it failed to set the reimbursement criteria. Its second attempt was again rejected by HIRA in July last year. At that time, Roche failed to secure reimbursement standards despite presenting additional results showing improved overall survival (OS) at the American Society of Clinical Oncology (ASCO) meeting. Meanwhile, the Polarix follow-up study of Polivy, presented at the American Society of Hematology (ASH) Annual Meeting 2024, is considered the first clinical trial in 20 years to expand the standard first-line treatment for DLBCL. Key results show that the Polivy combination therapy group demonstrated a clear improvement in overall survival (OS) compared to the control group treated with the existing standard therapy, R-CHOP. The lymphoma-related mortality rate was 9.0% in the Polivy combination therapy group and 11.4% in the R-CHOP control group. Approximately 5 years after treatment initiation, the risk of death in the Polivy combination therapy group decreased by 15%, an improvement over the previous 3-year follow-up result (6% risk reduction). In the case of Tecentriq, the 5-year follow-up study of IMpower010 showed that in patients with stage 2-3A non-small cell lung cancer (NSCLC) with PD-L1 expression ≥50%, who underwent complete resection and platinum-based chemotherapy, the Tecentriq adjuvant therapy arm resulted in an OS of 82.7%, which was significantly higher than that of the best supportive care (BSC) arm (65.3%).
- Company
- COVID-19 response more important for high-risk groups
- by Whang, byung-woo Sep 01, 2025 06:04am
- While COVID-19 has entered an endemic phase and become a resident disease in daily life, it remains a significant threat to high-risk groups like the elderly. In fact, the outbreak of COVID-19, which had slowed down for a while, has been showing an upward trend again. According to the Korea Disease Control and Prevention Agency's Infectious Disease Portal, signs of sporadic resurgence have emerged, such as the number of patients hospitalized for COVID-19 increasing from 139 to 220 within a week in early August. Hyun Jong Lee, Vice President of Academic Affairs, Korean Society of Otorhinolaryngology-Head and Neck Surgery (Director of Lee & Hong ENT Clinic), emphasized the importance of rapid diagnosis and treatment, stating, “Although public awareness has significantly declined since COVID-19 became endemic, the fatality rate remains high for high-risk patients if they become infected.” COVID-19 response remains critical for the elderly… proactive measures needed COVID-19 has shown a recurring pattern of outbreaks during the summer and winter seasons since entering the endemic phase. In clinical practice, the actual number of patients is reported to be higher than the official statistics. Lee explained, “Many hidden cases go undetected because COVID-19 is often mistaken for a common cold or air-conditioner-related illness. For those not considered high-risk, testing is not covered by insurance, so patients often don’t feel the need to get tested. In addition, many people who have had a previous infection assume, on their own, that ‘this time it can’t be COVID-19,’ which is not uncommon.” As a result, testing is carried out more actively only among patients aged 65 and older, those in poor general health, or when high-risk family members are living in the same household. Lee stated, “When recommending testing to suspected patients, only about 20-30% of those under 65 get tested, while about 50-70% of those aged 65 and older do.” He interpreted this as “likely because insurance covers diagnosis and treatment for those over 65, and they have greater health concerns.” Indeed, for those aged 60 and above, COVID-19 treatments are covered through reimbursement, allowing prescriptions to be issued upon a positive test result, which is why medical professionals actively recommend testing. However, for those under 60, both testing and treatment are out-of-pocket expenses unless specific risk factors exist, making it difficult for doctors to recommend them readily. Nevertheless, doctors unanimously agree that proactive measures are necessary for the elderly and those with underlying conditions who face a high risk of severe illness. According to Statistics Korea data, individuals aged 65 and older accounted for 91.9% of domestic COVID-19 deaths in 2022, and the fatality rate for those aged 65 and older was approximately 40 times higher than for those under 65. Lee emphasized, “For patients aged 65 and older, those with weakened immunity due to cancer, or those with serious underlying conditions, a COVID-19 infection still carries a very high risk of progressing to severe illness or death. When endoscopic examinations show clear signs that the condition may progress to bronchitis or pneumonia, physicians strongly recommend testing.” Paxlovid transforms COVID-19 response..."Contributing to Reduced Early Mortality" Currently, the mainstay of antiviral treatment for mild-to-moderate COVID-19 cases in Korea is Paxlovid (nirmatrelvir/ritonavir). Developed by Pfizer, this oral therapy has been proven effective when administered early in patients at high risk of severe disease progression. Lee evaluated, “Paxlovid is a very good drug that works by suppressing viral replication. It has demonstrated excellent effects when given in the early stages. Between 2023 and 2024, with stable supply and growing clinical experience, its use has expanded significantly, and we confirmed that it contributed to reducing early mortality.” The outstanding therapeutic effect of Paxlovid is also evident in patient experiences. According to Lee, “Elderly patients who had struggled greatly during previous COVID-19 infections now report a dramatic improvement after taking Paxlovid, saying they ‘feel much better.” Unlike when taking common cold medicine, patients reported feeling the effects directly after taking antiviral drugs, such as fever and pain subsiding much faster. These clinical observations are supported by research data. In the global EPIC-HR clinical trial, early administration of Paxlovid reduced the risk of hospitalization and death among COVID-19 patients by up to 86%. Similarly, a domestic study analyzing about 1.94 million patients reported that confirmed cases aged 60 or older who took Paxlovid within five days of symptom onset had a significantly lower risk of severe progression and death compared with those who did not. The prescription environment for oral COVID-19 treatments recently reached a major turning point. The government provided Paxlovid free of charge until last year, but starting this year, it transitioned to being reimbursed by national health insurance, meaning patients now bear part of the cost. Regarding this, Lee explained, “During the period when it was provided free of charge, some patients did not take the medication even after receiving a prescription because they didn't realize its value since they weren't paying for it. However, the shift from free to paid supply, introducing patient coinsurance, has brought one positive change: increased medication adherence.” “Reimbursement limited for the elderly and high-risk groups...Reimbursement criteria must be expanded” Nevertheless, this hasn't resolved all concerns on the site. Criticism persists that Paxlovid's current insurance reimbursement criteria are overly restrictive, potentially causing some high-risk patients to miss treatment opportunities. Lee emphasized, “Paxlovid currently faces many reimbursement restrictions. An infection that young family members might brush off lightly can be difficult for those in their 70s and 80s to recover from, potentially leading to death. Reimbursement should be lowered to include those aged 50 and above, and if safety is not an issue, it should also be expanded to include pediatric patients with underlying conditions.” In other words, experts believe that the current prescription system, centered on those aged 60 and above, should be expanded to include those in their 50s. They also advocate allowing healthcare providers to apply antiviral drugs at their discretion to middle-aged and older adults with underlying conditions. Finally, Lee urged high-risk COVID-19 patients and their families to adhere to two principles: prevention and prompt treatment. He advised, “As The Art of War states, ‘The best victory is one won without fighting,’ avoiding infection is paramount in COVID-19 management. Alongside hygiene protocols, establishing a primary defense line through vaccination in consideration of one's immune status is necessary.” Lee further added, “For those who cannot get vaccinated or fail to develop antibodies after vaccination, there is now Paxlovid. Since rapid diagnosis and treatment are crucial for its use, early testing and prompt treatment are a must for high-risk groups.”
- Company
- Capvaxive release imminent in the pneumococcal vaccine mkt
- by Whang, byung-woo Sep 01, 2025 06:03am
- MSD's adult-specific 21-valent protein-conjugated vaccine Capvaxive was granted marketing authorization in Korea by the Ministry of Food and Drug Safety and has emerged as a new competitor in the domestic adult pneumococcal vaccine market. With Prevenar 20 now launched in the market previously contested by Prevenar 13 and Vaxneuvance, Capvaxive is expected to target the market based on its unique selling point as an adult-specific vaccine with the broadest serotype coverage. Pic of Capvaxive Capvaxive is the first adult-specific vaccine designed to prevent invasive pneumococcal disease and pneumonia in adults. Unlike existing PCV vaccines targeting both children and adults, it covers a total of 21 serotypes, including 8 unique serotypes, reflecting the epidemiological characteristics of the adult population. This covers the serotypes responsible for 85% of invasive pneumococcal disease in adults aged 65 and older (based on U.S. data), and is expected to fill a prevention gap in the rapidly aging Korean market. Capvaxive’s influence has been growing steadily in global markets where it has already received approval. The U.S. Food and Drug Administration (FDA) approved Capvaxive in June 2024, and it is being quickly introduced on-site.. Based on MSD's announced earnings for this year, Capvaxive recorded USD 107 million in Q1 and USD 129 million in Q2, totaling USD 236 million (KRW 328.2 billion) in sales in the first half of the year. Considering it has been about a year since its full launch, its sales are expected to continue to grow. According to industry sources, MSD is expected to launch Capvaxive in the first half of next year. Considering how Vaxneuvance received approval in the second half of 2023 and launched in April the following year, its launch in Q1 is likely.. In this case, the adult pneumococcal market is also expected to undergo restructuring. Currently, the vaccinations available in Koera for adults include Prodiax 23 (PPSV23), Prevenar 13 (PCV13), Vaxneuvance (PCV15), and Prevenar 20 (PCV20). As Prodiax 23 is included in the National Immunization Program, effectively, three vaccines are competing in the non-reimbursement market. However, as Prevenar 20 and Capvaxive are the respective follow-up vaccines to Prevenar 13 and Vaxneuvance, the competitive landscape is expected to center on the competition between the two. As Chong Kun Dang partnered with Pfizer to distribute Prevenar 20 following Prevnar 13, the industry anticipates that Boryung Biopharma, which handles sales of Vaxneuvance, will distribute for Capvaxive in terms of continuity. Amid rising demand for preventive vaccines among the elderly and high-risk groups, attention is focused on whether the domestic introduction of Capvaxive will bring changes to future vaccine policies, such as Korea’s National Immunization Program (NIP), and market strategies. A vaccine industry insider explained, “With the balance shifting this year from Prevenar 13 to Prevenar 20 for adult pneumococcal vaccines, and now another vaccine with even more serotypes emerging, marketing competition to differentiate their product amongst the wide range of choices is set to intensify.”
- Company
- CKD says, "New ADC drug enters first clinical trials"
- by Son, Hyung Min Sep 01, 2025 06:03am
- Changsik Lee, Head of Chong Kun Dang Research Center Chong Kun Dang is accelerating its development of a new c-Met-targeted Antibody-Drug Conjugate (ADC). With its newly entered clinical candidate, 'CKD-703,' the company is aiming to secure a best-in-class ADC and is seeking a differentiated strategy in a globally competitive landscape. On August 29, Samsung Medical Center and Aimed Bio co-hosted the 3rd ADC Conference at Samsung Hospital's main auditorium. Chong Kun Dang's research director, Changsik Lee, introduced the ADC new drug candidate CKD-703, which has entered the clinical stage. Since 2019, Chong Kun Dang has been collaborating with the Dutch company Synaffix on ADC discovery. In February of last year, it acquired non-exclusive rights to Synaffix's ADC platform technology in a deal valued at $132 million. Synaffix is currently partnering with several key domestic bio-companies on its platform technology. Using this technology, Chong Kun Dang successfully developed CKD-703. CKD-703 is a drug that targets the hepatocyte growth factor receptor (c-Met), which is overexpressed in cancer cells, and is being developed for solid tumors, including non-small cell lung cancer (NSCLC). Last month, the U.S. Food and Drug Administration (FDA) approved Chong Kun Dang's Investigational New Drug (IND) application for a Phase 1/2a clinical trial of CKD-703. This is the first time Chong Kun Dang has entered a global clinical trial for an ADC. c-Met targeted by CKD-703 is a protein expressed by the epithelial-mesenchymal transition (MET) gene. As a key signaling protein, c-Met is a well-known oncogene linked to the development of various solid tumors, including NSCLC, colorectal, gastric, and liver cancers. It is known that the c-Met mutation occurs in 6% of NSCLC patients. Currently, AbbVie's Emrelis (telisotuzumab vedotin) is the only commercially available ADC that targets the c-Met mutation. Emrelis received accelerated approval in the U.S. in May for NSCLC. In its Phase 2 clinical trial involving 84 patients with c-Met overexpression, the objective response rate (ORR) was 35%, and the duration of response (DoR) was 7.2 months. Lee noted, "In clinical trials, Emrelis's efficacy was primarily observed in patients with c-Met overexpression, with an ORR of 35%. We believed there was a significant unmet need that could be addressed in patients with low expression and in terms of response rates." Synaffix (Netherlands) However, competitors continue to emerge. Celltrion is also focusing on c-Met-targeted ADCs, having received approval for its CT-P70 Phase 1 clinical trial in Korea and the U.S. this year. Chong Kun Dang plans to differentiate itself from global competitors in areas such as toxicity management and treatment response rates to prove its commercialization potential. Lee stated, "We have confirmed that CKD-703 has a high yield of over 85%, which poses no issues for its progression to clinical trials," and added, "The payload was the main concern, but there was no reason to change it from the one used in Elahere to achieve a better effect. We used MMAE, a microtubule inhibitor payload, which is also applied in Emrelis." And added, "CKD-703 has the potential for improvement over existing treatments in terms of efficacy and safety." Lee analyzed, "In preclinical monkey models, the incidence of thrombocytopenia, a condition where platelet counts drop below the normal range, was lower for CKD-703 compared to Emrelis." Lee emphasized, "We are dedicating our utmost corporate resources to the development. In this clinical trial, we expect CKD-703 to demonstrate a higher efficacy than existing treatments in patients with c-Met overexpression, and also prove its efficacy in patients with low and intermediate expression. Our goal is to secure a best-in-class treatment, and we are open to the possibility of combination therapy with immunotherapies."
- Company
- Ozempic adds indication for patients with diabetes and CKD
- by Son, Hyung Min Aug 29, 2025 06:07am
- Novo Nordisk Novo Nordisk Korea (General Manager: Kasper Roseeuw Poulsen) announced on the 28th that Ozempic has been approved as a treatment for type 2 diabetes with chronic kidney disease. With this approval, Ozempic is now indicated for: ▲ Use as an adjunct to a two-drug regimen (either alone or in combination with other diabetes medications), in adults with type 2 diabetes whose condition is not adequately controlled. ▲Reducing the risk of major adverse cardiovascular events (MACE) in adults with type 2 diabetes and confirmed cardiovascular disease. This approval expands its indications to encompass the broadest patient base among GLP-1 receptor agonist (GLP-1RA) type 2 diabetes treatments launched in Korea. Ozempic has further solidified its position as a comprehensive treatment option for adult patients with type 2 diabetes, not only offering glycemic control but also a reduction in the risk of major adverse cardiovascular events (MACE), sustained reduction in estimated glomerular filtration rate (eGFR) in adults with type 2 diabetes and chronic kidney disease, and reduction in the risk of reaching end-stage kidney disease and cardiovascular disease mortality. The expansion of Ozempic's indication to chronic kidney disease was based on results from the FLOW study. The FLOW study was a multinational, multicenter, placebo-controlled, double-blind, 1:1 randomized study evaluating the efficacy and safety of Ozempic versus placebo in 3,533 adult patients with type 2 diabetes and chronic kidney disease. The primary endpoint of this study was a composite endpoint consisting of a sustained 50% or greater decline in estimated glomerular filtration rate (eGFR) from baseline, occurrence of end-stage kidney disease, or death due to cardiovascular or renal disease. The median follow-up period was 3.4 years, and the Ozempic group demonstrated a 24% reduction in the risk of the composite endpoint compared to the placebo group. Furthermore, serious adverse events reported in this study occurred in 877 patients (49.6%) in the Ozempic group and 950 patients (53.8%) in the placebo group, showing similar rates between the two groups. Kasper Roseeuw Poulsen, General Manager of Novo Nordisk Korea, stated, “The expansion of Ozempic's indication for chronic kidney disease marks a turning point, signaling that diabetes treatment has entered a new era where treatment goes beyond simple blood glucose control to comprehensive management of cardiovascular and renal risks. “As a company that has led diabetes care for over 100 years, Novo Nordisk is setting new standards in type 2 diabetes treatment based on its scientific innovation and accumulated experience. Moving forward, Novo Nordisk will continue to serve as a trusted partner for patients and healthcare professionals across all areas of diabetes care, and lead the future of integrated treatment in diabetes.”