- LOGIN
- MemberShip
- 2025-12-19 05:34:46
- Product
- It costs ₩867 million to become a physician
- by Kang, Shin-Kook Feb 11, 2020 06:29am
- It is estimated that it costs ₩867 million from entering medical school to obtaining professional qualification. The Korean Medical Association(KMA)'s research institute for healthcare policy (Director Deok-sun Ahn) announced on the 7th that it has published a research report on the estimation of doctor training costs and public support. According to the research report, the average cost of education per student in the basic medical education stage (1 year of premedical course and 1 year of regular course) was ₩64.97 million (minimum ₩54.12 million, maximum ₩77.62 million). Divided into premedical courses and medical courses, the average annual education cost per premedical student was ₩25.3 million (at least ₩14.93 million, up to ₩38.81 million). The average annual education cost per medical student was ₩39.95 million (minimum ₩3257 million, maximum ₩48.51 million). As a result of estimating the cost of internships, the average annual training cost per intern was ₩73 million (min. ₩55.5 million, max. ₩93.95 million). As a result of estimating the training costs of majors (5 departments: internal medicine, surgery, obstetrics, pediatrics, family medicine, etc.), the average annual training cost for each major is ₩146.04 million(minimum ₩111.18 million, max. ₩187 million). According to the results of estimating the cost of each doctor's training, the total cost of training one doctor (to become a specialist) is ₩887 million, and the total annual cost for training doctors is ₩2.7175 billion. On the other hand, in the US, UK, Germany, Japan, and Canada, the state and society share a large part of the cost of physician training. In the United States, for example, the share of medical costs needed to train doctors is shared by various organizations, including 23% in the state, 8% in federal research funds, 18% in medical schools, 28% in clinical care, and donations. According to the U.S. medical schools, 70% of the per capita training costs are shared by Medicare (20% direct, 50% indirect) and 30% by Medicaid and other private health insurance companies. Korea's budget is ₩72 trillion (₩60 trillion in social welfare and ₩12 trillion in health care) in 2019, and in the budget, the portion for training and aptitude and supply of medical personnel is ₩24.9 billion, of which, the budget for support for nurturing majors and qualification examinations for specialists is about ₩1.3 billion. The Research institute for healthcare policy said, "As the social interest in patient safety increases, the education and training environment for excellent doctor training is increasing, the study was meaningful in estimating the cost of training and total cost per doctor and suggesting the justification for public support". The research institute said, “Based on the results of the study on the estimation of the cost of doctor training and public support measures, it is necessary to form a consultative body for social discussion of the cost sharing of doctor training, and social councils should involve the important stakeholders in health care, the nation, the Korean Medical Association, medical schools, intern & training Centers, local governments, and the general public”.
- Product
- Lyxumia by Sanofi stops supply in the second half
- by Kim, Min-Gun Feb 10, 2020 06:31am
- Sanofi-Aventis KoreaSanofi-Aventis Korea's supply of GLP-1 analog diabetes treatment ‘Lyxumia (Lixisenatide)’ will be discontinued from June this year. According to the distribution industry on the 7th, Sanofi recently announced that it has stopped supplying two formulations, such as 10μg and 20μg of Lyxumia pen, due to company circumstances. Sanofi said in an announcement that it is scheduled to withdraw its product license (from the Ministry of Food and Drug Safety) for the convenience of the company. If Lyxumia is withdrawn in March, the supply is expected to cease in June this year, when inventory held by wholesalers is exhausted. The remaining benefit period is expected to be up to six months from the date of withdrawal. As a result, benefits are expected to cease from October this year. The reason for the withdrawal of Lyxumia is unknown. However, the rapid decline in revenue after the advent of competitive products is believed to have had some effect on supply disruption. Licensed as a GLP-1 analogue in March 2013 by the Food and Drug Administration, the product was approved for glycemic control, diet and exercise therapy supplements in adult patients with type 2 diabetes. At the time of the market, once-daily administration showed attention for lowering blood sugar regardless of pre and post meal. However, Lixumia's sales declined sharply in 2016 because of Trulicity (Dulaglutide), which is administered once a week with the same GLP-1 analog family. According to drug market research institutes such as IQVIA, The sales fell to ₩500 million in 2018 and ₩300 million in 2019. The poor performance of the family of GLP-1 analogs administered once a day is not the only Lixumia. Victoza (Liraglutide) by Novonordisk and Byetta(Exenatide) by Astrazeneca also fell below ₩100 million. At present, the market size of the GLP-1 analogue is known to be about ₩30 billion. The industry believes that it is due to the convenience of once-weekly administration and Trulicity, which is recognized for its combination with basal insulin at the end of 2017. The long-lasting product has reduced the rejection of injections. This is a long-lasting product that has increased convenience. The market grew, but as Trulicity encroached, once-daily dosing products gradually disappeared.
- Product
- Taltz for interleukin Psoriasis expands Rx Area in hospitals
- by Eo, Yun-Ho Jan 06, 2020 06:22am
- Lilly's interleukin IL-17A antagonist, Taltz, is settling on its prescription area at general hospitals. According to the related industry on the 6th, psoriasis treatment Taltz (Ixekizumab) passed the drug commitee (DC) of Seoul National Hospital recently after Asan Hospital and Severance Hospital among the Big Five. In addition, prescription codes have been made into major hospitals such as Seoul National University Bundang Hospital and Pusan National University Hospital. Taltz, which was released in late 2018, was slower to be priscribed in general hospitals compared to Janssen's IL-23 antagonist Tremfya (Guselkumab), which had entered the same period. Novartis' Cosentyx (Secukinumab) which has the same mechanism as Taltz, entered the market earlier. For this reason, Taltz had difficulty, but it is gradually expanding being prescribed. Accordingly, the issue is how the competition between interleukin drugs in the psoriasis treatment field will change. Lilly has also published the latest IXORA-R study in patients with moderate-to-severe plaque psoriasis , which directly compares the efficacy of Tremfya and Taltz. As a result, the Taltz-administered group achieved a statistically significant level of skin improvement compared to the Tremfya-treated group, which achieved 41.3% of patients with complete clean skin (PASI 100), the primary endpoint at week 12. All major secondary endpoints were also met. The reaction rate were higher than Tremfya at the 2nd PASI 75 ratio, the 4th and 8th PASI 90 ratio, the 4th and 8th and 24th PASI 100 ratio, the 12th week's static Physician's Global Assessment (sPGA) 0 ratio, and the 1st PASI 50 ratio. Andrew Blauvelt, Ph.D., working at the University of Oregon Research Center, USA, said, “The results of IXORA-R showed that Taltz is an effective treatment that helps more patients reach complete clean skin at week 12 of treatment, as early as the first week of treatment, skin plaque lesions improved by 50%”. In the IXORA-R clinical trials, the safety profiles of Taltz and Tremfya were similar to those reported previously. Lilly plans to announce in 2020 the results of the 24th week PASI 100 attainment rate, one of the key secondary endpoints for the IXORA-R trial.
- Product
- Samsung completes Phase III of Lucentis Biosimilar
- by Lee, Seok-Jun Jan 02, 2020 06:08am
- Samsung Bioepis’ Lucentis Biosimilar (SB11)' phase III completed. Lucentis' global sales last year were about ₩4.2 trillion. Samsung Bioepis recently announced its last patient visit for the SB11 phase III trial was done. Phase III examined compared non-inferiority with the original Lucentis in a total of 705 patients with Wet Age-related Macular Degeneration from Mar 2018. Lucentis is a treatment for eye diseases such as macular degeneration and diabetic macular edema, jointly developed by Roche’s subsidiary Genentech and Novartis, multinational pharmaceuticals. Samsung Bioepis plans to announce the results of phase III next year and proceed with the marketing authorization application process in Europe and the United States. SB11 is Samsung Bioepis' first eye disease treatment project. Meanwhile, Samsung Bioepis recently signed marketing partnerships about two types of eye disease treatment pipelines (SB11: Lucentis Biosimilar, SB15: Ilia Biosimilar) with Biogen, including the US and Europe. SB15 is a case that recognized market value from partners even though it is a candidate for clinical preparation.
- Product
- Oral treatment by Sanofi has the highest number of defects
- by Kim JiEun Dec 26, 2019 06:30am
- Ten defects were found in the same product of the same company a year The Seoul Pharmaceutical Society (Chairman Dong-ju Han), the pharmacy committee (Vice chairman Yong-seok Choi, Head Commissioner Woo-young Chang, Soo-hyun Byun, and Tae-seok Kang), and the Pharmacist Guidance Committee (Chairman Kyung-Jin Jeon and Soo-Yul Lim) announced the results of the submission of bad drugs this year. The Seoul Pharmaceutical Society has received reports from member pharmacies since this year by promoting the operation of the bad drugs report center. According to the Seoul Pharmaceutical Society, a total of 174 cases were received and processed at 70 member pharmacies in 24 Seoul branches. The most common types of defective drugs received were simple damages (33 cases), including ▲shortage of 17 cases, ▲poor PTP packaging, ▲12 poor tablets wear, ▲8 poor inspections, ▲8 poor tablet and poor coating, ▲ Seven PTP and bad inspection ▲4 bad inspection, ▲4 bad sealing, ▲4 bad containers, ▲Three poor granulation and bad tablets. In addition, there were discoloration and change of appearance, mixing of other products, mixing of foreign materials, poor filling, and excessive quantities. The Seoul Pharmaceutical Society received the most reports with 10 cases of oral infectious drugs by Sanofi. It recently announced that a recovery action was requested for the serial number and expiration date. In addition, a total of 136 items were reported. The Seoul Pharmaceutical Society receives a reception for defective drugs through text messages (Tel: 010-3568-5811), and the member pharmacy receives the name of the department, the name of the pharmacy, the name of the pharmaceutical company, the name of the drug, the manufacturing number/expiration date, and the bad drug picture. The pharmacy society asked for information from member pharmacies as it could be used as a basis for data accumulation even when pharmacies and pharmaceutical companies (wholesalers) provided their own drugs. They also pointed out that even though there are many cases of drug shortages in pharmacies, it is not easy to pass on because of lack of proof. In this case, they also asked for active reporting. Chairman Dong-ju Han said, “Defective drug notification serves as an important stepping stone to improve the quality and to ensure the safety of patients by identifying the cause and preventing recurrence and awaits the participation of member pharmacies”.
- Product
- “Lipitor not sold out”, Pfizer clarifies rumors
- by Kim JiEun Dec 18, 2019 06:24am
- Rumors of cholesterol lowering treatment Lipitor tablet going out of stock have been spreading throughout major pharmacies, but its company clarified the rumor is false. Pfizer Upjohn Korea on Tuesday told Daily Pharm that the manufacturing of Lipitor 10 mg tablet (28BLP/ 90BLT) has no issue at the moment. The company’s statement was an answer to the recent Lipitor stock-out rumor spreading among pharmacies. Apparently, the talk of Lipitor going out of stock until next year was looming from pharmacists on social media channels and triggered some pharmacies to start hoarding the product. With a temporary spike in order volume, a certain distributor had to notify their salespeople to explain about the situation of pharmaceutical distributors and online shops having scarce stock of the drug to their designated pharmacies. The distributor informed their salespeople that “Lipitor has been sold out due to a baseless false rumor. It has affected all distributors in Korea. As temporary demand in other products has been increasing as well, please visit designated pharmacies to explain the situation”. The whole situation has caught the global company by surprise. The company has been ordinarily manufacturing and supplying the item, when the order has exponentially hiked because of the rumor. Pfizer Upjohn Korea official told, “As of afternoon of Dec. 16, Lipitor 10 mg supply is in a normal state. The company has confirmed Lipitor’s stock in some pharmacies have been sold out. To minimize inconvenience of doctor, pharmacist and patient, the company is in process of studying the origin of the rumor and resolving it”. The company also explained the drug is supplied based on market demand projection and it notifies the public when it predicts drug stock-out from plausible cause. “The company provides products considering a projection based on multiple years of collected market supply and demand data. Accordingly, supply amount is regularly monitored. When the company expects a setback in supply process, the company informs organizations of distributor and pharmacist to minimize turmoil in the market”, the official elaborated. “Pfizer Upjohn Korea would continue to strive for stable supply of drug”, the company official added.
- Product
- Metformin impurity amount, theoretically 1/90 of Ranitidine
- by Kim, Jin-Gu Dec 10, 2019 06:31am
- Molecular structure of MetforminWith Metformin, issues of impurity detection have been raised, and it is possible that N-nitrosodimethylamine (NDMA) will be detected much less than earlier Ranitidine or Nizatidine. According to officials at the MFDS on Dec 9, a qualitative structure activity relationship (QSAR) is used as a simple test to determine the toxicity of a substance. It is a test method that compares and predicts physical and chemical properties by similarity of intrinsic chemical structure. Since the properties of a substance are closely related to its molecular structure, it is a principle that similar physical structures have similar physical properties and toxicity. In fact, the results of predicting the toxicity of Ranitidine-Nizatidine in this way are nearly identical to the actual detection of NDMA in both formulations, according to the MFDS official. According to the official, the results of Ranitidine and Nizatidine QSAR were 90 and 5, respectively. The actual detection amounts were 53.50 ppm and 1.43 ppm based on the maximum values. A MFDS official said, "On this extension, Metformin's QSAR test results are only around 1." It is expected that the possibility of actual detection is much lower than that of Ranitidine or Nizatidine. ◆'Ranithidine 90%, Nizatidine 5%, Metformin 1% or less' This is in line with the findings of Agilent, a US material analysis company. Agilent conducted an experiment in May 2017 to investigate the effects of discarded drugs and chemicals on water pollution. Agilent66 substances were selected for the survey. It includes Ranitidine and Nizatidine, and Metformin. Among the various substances, it is explained that a substance that is likely to be transformed into DMA (dimethylamine) is selected. DMA, together with nitrite, is one of the two materials of NDMA Experimental results showed that the rate of NDMA formation was 60-90% for Ranitidine. Of the 100 ranitidine molecules, 60 to 90 change to NDMA. 5% to Nizatidine. Metformin was found to be less than 1%. This is consistent with the description of the MFDS official. However, as in the case of Valsartan, it is not possible to exclude the possibility that NDMA is generated due to reaction of a specific solvent in the manufacturing process. In the case of Valsartan, dimethylamine was formed as a decomposition product during the high temperature process of dimethylformamide (DMF), which was used as a solvent, and the dimethylamine reacted with nitrite under acidic conditions, leading to NDMA formation. An official from the MFDS explained, “It is difficult to identify the cause of NDMA in Metformin right away, and we have to chop up the molecules of Metformin into small pieces to closely examine the similarity with Ranitidine and the toxicity of the substance itself”. "As a result of the QSAR test, Ranitidine was 90 and Metformin was 1, and A detailed survey of Metformin will be performed and it will be compared with the QSAR test results" said the official.
- Product
- The Minister Lee, Enhanced post-de-factor management
- by Jung, Heung-Jun Nov 18, 2019 10:21pm
- Lee Eui-kyung, the Minister of MFDS has released a long-term follow-up plan to strengthen the post-de-factor safety management system. On the 15th, the Minister Lee attended Korean Academy of Social & Managed Care Pharmacy and presented the four directions of drug safety management in four categories: patient safety, accessibility, safety ecosystem, and globalization. In particular, the Minster Lee emphasized that she will lead to a paradigm shift centered on patients by strengthening post-de-factor management. She said, "In the meantime, in the event of Invossa’s case , it was most of the time that the license was revoked or changed. In the future, we will thoroughly control the side effects, operate the long-term follow-up management system, and carefully regulate the damage. I will lead the paradigm. " For 3,000 patients for injecting Invossa, she will select 20 medical institutions and conduct a long-term follow-up survey for 15 years. "We have registered for 80 to 90 percent of our patients." she said, "Patients have used 400 medical institutions, but the concern is about cancer. So long-term follow-up should be in a hospital with oncology." "We plan to track them all at large hospitals, and we will also carry out causality research of cancer." On this day, the “Long-term patient tracking survey system (Draft)”, which will be applied to future risk medicines, was announced. According to the plan, when the MFDA issues a follow-up order, companies make a plan and MFDA goes through approval process. Companies conduct follow-up investigations. Causality evaluations and follow-up actions are conducted with the MFDS. The Minister Lee said, "We still have to be specified liability compensation. The government thinks that product defects should be shouldered by pharmaceutical companies. But, pharmaceutical companies have different positions that they are not responsible for what they didn't know at the time. We are discussing the responsibility with the relevant committees” ◆Strengthen practical use of RWD (Real World Data), R &D investment extension. In addition, MFDS will strengthen the use of RWD (Real World Data) and RWE(Real World Evidence) next year to strengthen patient safety management It plans to establish big data utilization system such as clinical site RWD and RWE in clinical trial RCT. To this end, it will actively invest in R & D costs. She said, “We will make an official announcement in January about the plans for using big data. MFDS R & D expenses rose from ₩85 billion to ₩100 billion this year. But most of them are the cost of experimentation. I'm going to turn this into RWD study”. She also announced that she will soon disclose ways to improve safety for conditional phase3 permits. "There is a lot of controversy about whether a conditional phase3 permit will guarantee the patient first, or if he will give the opportunity after ensuring full safety, if there is no alternative." We will soon release to the media on how to make it work more securely. ” ◆As new drug development countries become more important, permit strengthening of MFDS expertise She said that as Korea is actively developing new drugs, licensing is becoming more important, so the MFDA will also strengthen its expertise. She said, “If the drugs were reviewed at least once because foreign new drugs were introduced into the country in the past, currently there is a need to be more burdensome in terms of initial authorization and to reinforce professionalism since new drugs are being developed in Korea. Only about 350 people are screened by the MFDS, but there are 6,000 Ph.D in US. The MFDS will focus on strengthening its expertise in the future.” She also said “MFDS or pharmaceutical companies can’t do it alone, Infrastructure maturity must increase” "The long-term policy is needed to fundamentally improve the problem," She said. It is true that there is a lack of policy research think tank, ” She also recognized the necessity of analyzing and evaluating the future system.
- Product
- 7 out of 10 doctors exposed to patient violence
- by Kang, Shin-Kook Nov 15, 2019 06:29am
- Apparently, seven out of ten doctors have faced verbal abuse or physical violence against them by a patient or their family. On Nov. 13, Korean Medical Association (KMA, President Choi Dae-zip) published a result of an urgent survey conducted on 2,034 member doctors of the organization. The survey result found among 1,455 doctors (71.5 percent), who have experienced verbal abuse or physical violence against them committed by a patient or their family in exam room in last three years, about 15 percent of them have been physically assaulted, and 10.4 percent ended up with a physical damage. KMA President Choi Dae-zip And more than a half of the surveyed doctors said they have experienced verbal abuse and physical violence against them about once or twice a year. 9.2 percent answered they have been in such situation at least once a month. Most of such aggression was fired up by medical exam results, and some other cases were sparked from complaints of long waiting time and expensive medical service charges. Moreover, 61 percent of the doctors answered a patient or their family came back to the abused doctor later for medical service. 1,254 doctors (61.7 percent) among them have also been forcefully demanded by a visitor to forge a medical report or falsely edit already issued medical record. “The association aims to protect member doctors by introducing a new bill that stipulates punishment on visitors demanding doctors to forge medical record. KMA is committed to have the lawmakers to promptly legislate bills to protect doctors in vulnerable position. The association has been constantly urging the law turning down an indictment for committed assault in healthcare institute when a victim drops the charges should be abolished, and also doctor’s rights to refuse patients should be legally justified. The latest survey has provided us enough concerning evidences,” said KMA official. Moreover, KMA officials pointed out “The survey result says only 6.9 percent of the doctors at the moment have a protected space or facility to avoid verbal abuse and physical violence in an exam room. It means most of the doctors are vulnerably exposed to the harmful threats. The government should pay more attention on creating a safer environment for healthcare institutes”.
- Product
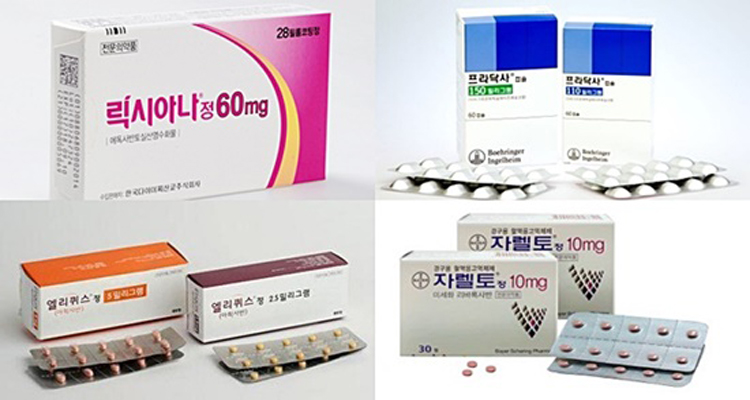
- Lixiana joins NOAC market race late but takes the lead
- by Byun Kyung A Oct 31, 2019 09:59am
- (Clockwise from upper left) Images of Lixiana, Pradaxa, Xarelto, and Eliquis A follow-on drug in the non-vitamin K antagonist oral anticoagulant (NOAC) market, Lixiana, continues to lead the market. The treatment took the lead from Xarelto in last January for the first time, and it has been the most prescribed drug in the market for nine consecutive months. Pfizer and Bristol-Myers Squibb’s Eliquis had to face a Korean-made generic as a competitor, but its sales performance was unaffected with its original price maintained. On Oct. 28, UBIST, a pharmaceutical market data research firm, reproted Lixiana (edoxaban) marked an accumulated prescription of KRW 40.1 billion in the third quarter. The figure skyrocketed by 68.5 percent from last year same period. Lixiana recorded prescription sale of 14.9 billion won in the third quarter only and marked its record-breaking quarterly performance. The treatment reached outpatient prescription sale of 4 billion won in last January and beat Xarelto’s (rivaroxaban) prescription sale of 3.8 billion won, for the first time. The top spot in the NOAC prescription market has been solidified for nine months straight. The total sale gap between Lixiana and Xarelto’s prescription marked 6.6 billion won and was widened in the third quarter, due Xarelto’s sale dropping 0.2 percent in the third quarter than the year before. The treatment was ranked on second place in the market with 33.5 billion won accumulated prescription sale. Outpatient prescription sales trend of four NOAC items by month (Unit: KRW1 million) Source: UBIST Both internal and external factors played a part in shuffling the NOAC market rank. Bayer’s German production plant issue prolonged the unstable supply management in Korean market, and also the revised pharmaceutical product labeling regulation required all dosage containers to be reprocessed and slowed down Xarelto’s come back. In the meantime, Daiichi Sankyo and Daewoong’s co-promotion revved up the prescription sales of a follow-on drug, Lixiana. Insider from Daiichi Sankyo evaluated, “The healthcare providers and patients were positive about Lixiana’s better drug compliance with once-daily regimen, and improved safety for patients backed by Phase 3. The long-lasting co-promotion partnership with Daewoong, started from Sevika HCT deal, also created a great synergy effect boosting the sales performance”. Monthly prescription sales trend for Eliquis and apixaban generics (Unit: KRW) Source: UBIST Eliquis cumulated 31.3 billion won prescription sales this year, showing 32.1 percent surge than the year before. As of the third quarter, difference in accumulated prescription sales between Xarelto and Eliquis is only about 2.1 billion won. Eliquis was the first NOAC original to compete against generics, but its sales were not influenced as it maintained its original price. After the Korean Supreme Court in last June invalidated drug patent protecting Eliquis, the drug now has Korean-made generics competitors from Chong Kun Dang, Yoo Young, Yuhan, Huons, and Hanmi. But as Ministry of Health and Welfare has decided to defer Eliquis’ price reduction until the final decision on the treatment’s substance patent case is to be made on coming Dec. 31. Currently, the drug still maintains its initial price of 1,185 won per tablet. On the other hand, Chong Kun Dang’s competing generic with the highest content apixaban is about half the price of Eliquis. Boehringer Ingel Heim’s Pradaxa (dabigatran) marked accumulated prescription sales of 12.4 billion won in the quarter. The company sought for a significant increase in market share with a co-promotion deal with Boryung, but the sales performance did not show much increase than last year with 12.3 billion won.