- LOGIN
- MemberShip
- 2025-12-17 23:28:31
- InterView
- “Allowing safe use of JAK inhibitors over restrictions"
- by Eo, Yun-Ho May 26, 2022 05:56am
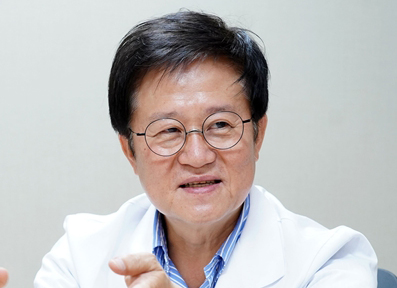
- Professor Ki-Jo Kim Nowadays, safety is as important as a drug’s efficacy. In particular, reaffirming the safety of drugs that require long-term administration post-approval is essential. JAK inhibitors, which have entered the market as an oral option to treat various autoimmune diseases such as rheumatoid arthritis and ankylosing spondylitis, have been embroiled in controversy since last year. In 2021, the US FDA had warned of increased risk of cardiovascular events and cancer on the use of Jak inhibitors, and the MFDS also issued a Dear Healthcare Professional Letter on the issue. In the end, the FDA decided to include the increased risk of major heart-related events, thrombosis, and death in the black box warnings. The risk is undoubtedly an issue that cannot be overlooked. The risk of cardiovascular disease is a common issue that tags all treatments used for chronic diseases, but more accurate judgment on this risk is needed for JAK inhibitors as some patients do certainly benefit from its use. Also, various factors including age and race need to be investigated. On this, Ki-Jo Kim, Professor of Rheumatology at Catholic University St. Vincent Hospital, lectured on the ‘'Management of Rheumatoid Arthritis considering Safety” at the KCR 2022 (the 42nd Korean College of Rheumatology Annual Scientific Meeting and the 16th International Symposium) that was held from the 19th to 21st. Dailypharm met with Professor Kim to hear about the efficacy and safety of JAK inhibitors. -Could you tell us about your lecture? When first introduced, JAK inhibitors opened a new paradigm in the treatment of rheumatoid arthritis. Its safety issue had risen last year with the announcement of the Oral Surveillance study results that showed that JAK inhibitors may increase the risk of some cardiovascular disease and cancer. This prompted a fierce debate in academic societies in the US, Europe, and Korea. I have reviewed and reevaluated relevant data, studied how to read the results and interpret the studies that followed, and investigated whether different JAK inhibitors showed different results for the presentation. -What was your conclusion? When looking into the ORAL Surveillance results in detail, data showed that the JAK inhibitors had a slightly higher risk of developing cardiovascular disease and cancer compared to TNF inhibitors. But in detail, even this slight difference was limited to North American patients aged 65 or older. Also, supplementary data in the paper showed that a higher proportion of North American patients in the study had a high risk of developing cardiovascular disease, due to factors such as being over 65 years of age, obesity, high blood pressure, diabetes, and various drugs intake, etc. In this sense, the high-risk patients in the North American patient group may have driven such results. The real-world data that followed studied all patients with rheumatoid arthritis. No difference was found in these patients, and there was no difference when patients aged 50 or older that had 1 risk of cardiovascular disease were extracted and investigated as in the ORAL Surveillance study. A tendency was there, but with no statistically significant difference. When considering these factors, the ORAL Surveillance study data may have shown a larger difference due to its various limitations and regional variations. .The FDA seems to be quite strict in monitoring the risk of cardiovascular events .I understand that it is that much of a serious issue, but also suspect the decision made for JAK inhibitors had been somewhat influenced by this tendency. I agree to a certain extent .Moreover, due to the recent COVID-19 pandemic and the rapid introduction of vaccines, many patients seem to have become more sensitive about safety, not only for the vaccines but for all drugs in general .This may be why the FDA made such preemptive measures based on just one study result .-The rising concern is that due to the issue, authorities may limit the prescription of JAK inhibitors to ‘patients that are unable to use Anti-TNF therapies.’ What is your opinion on this? It’s a shame .JAK inhibitors have the advantage of being an oral formulation .There is stress in receiving regular shots, especially in the case of intravenous agents .There are patients who clearly show an effect when prescribed JAK inhibitors .It’s not that the anti-TNF therapies are lacking in effect, it’s just about what better fits the patient .Some patients who seem fine and are well controlling their inflammations but complain of pain or bad conditions were relieved of their inconveniences with JAK inhibitors .All drugs have adverse reactions that are identified with the use in their respective indications .We carefully examine these to select an appropriate drug for each patient .I believe it is better to let the doctors in the field use their discretion for the safe use of JAK inhibitors rather than impose regulations and restrict its use to the second-line.
- InterView
- We are making all efforts to reimburse ‘Vyndamax’
- by Eo, Yun-Ho Apr 21, 2022 06:03am
- 김희정 전무 Rare disease patients suffer more due to the 'rarity' of their condition. Due to the small number of affected patients, even drugs that are already available cannot be easily listed for reimbursement due to difficulty in demonstrating cost-effectiveness or predicting their fiscal impact on the NHI budget. Rare diseases are diseases that affect fewer than 20,000 people or those for which the number of affected patients cannot be estimated due to difficulties in diagnosis. As rare diseases are difficult to diagnose and treat and have a significant impact on the life expectancy of the patients, it is imperative to ensure patient access to the treatments. However, due to the small number of affected patients, initiating clinical trials for such treatments in itself is quite difficult. In this context, Pfizer's Rare Disease Busines Unit has been currently exerting all its efforts to list its ‘Vyndamax (tafamidis 61mg),’ a treatment for ATTR-CM (ATTR amyloidosis with cardiomyopathy) for reimbursement in Korea. However, the process has not been so smooth. The company has already failed twice and is now making its third attempt at reimbursing the drug. Dailypharm met with Hee-Jeong Kim, Rare Disease Lead of Pfizer Korea to hear about the company’s position and state of affairs. -In Korea, it is not an exaggeration to say that reimbursement determines the success or failure of a new drug. As much as the government provides good coverage for the reimbursed drugs, this also makes it more difficult for drugs to be listed for reimbursement. Have you felt this while leading the Rare Disease BU at Pfizer? Korea’s strength is in its coverage (range of coverage). Patients in Korea can receive broader benefits in the course of their treatment due to Korea’s consistent policy and predictable supply under the single-payer system, once the drug is listed. Other markets abroad operate under various schemes. Access to new drugs is a little more difficult in Asia than in other regions. In Europe, new drugs are introduced at a relatively faster rate. In Spain, separate funding is operated for each region, and in the UK, the NHS has allocated alternative funding schemes for necessary drugs. The prompt introduction of new drugs is indeed difficult in Korea due to the wide scope of use of NHI finances from the patient’s perspective. The advantages of the single-payer system that I mentioned previously are partially offset by the strict standards set for new drugs. -This is the third attempt at reimbursement for ‘Vyndamax.’ It seems that the company’s determination will be as important as the government's will in obtaining the reimbursement approval. What efforts have Pfizer been making for the reimbursement?’ All employees at Pfizer Rare Disease have a strong desire to deliver the value of Vyndamax’ to patients in Korea. We plan to continue making attempts through various tracks available in Korea. ATTR-CM is a rare disease that lacks studies and trials to identify the exact prevalence of the condition itself. We fully understand the government’s concerns regarding this ambiguity and have continuously been in discussions with our head office to prepare an innovative risk-sharing plan as requested by the government. However, the agenda has not even passed the Drug Reimbursement Standard Subcommittee, therefore, we are not at the stage to discuss the risk-sharing plan being prepared by the company. We did not expect the drug to fail at setting the reimbursement standard stage so many times, therefore, our prime focus is on passing the Drug Reimbursement Standard Subcommittee this time. We will continue exerting our utmost effort at every stage that follows. -What about collaboration with academic societies and patient groups? The academic societies and patients groups recognize the need for the prompt reimbursement of ‘Vyndamax’ and have expressed their opinion to the government several times. Due to the small number of affected patients, it takes a significant time for HCPs to accumulate the expertise required. The Rare Disease BU cannot exist if the company only considers performance and numbers. In this perspective, having a sense of mission seems to be more important in our line of work. Our drug is necessary for further diagnosis of rare diseases, and we are sorry that there is no treatment currently available for use for HCPs and patients in Korea after the patients are diagnosed with ATTR-CM.
- InterView
- "Will make R&D partnerships, introduce new drugs in Korea"
- by Apr 14, 2022 05:56am
- The China Shanghai-based Antengene has started its activities in earnest in the domestic pharmaceutical market. Marking its start with Xpovio (selinexor), a blood cancer drug that was approved in July last year, the company aims to introduce more new drugs in cancers with high unmet needs. Antengene is an anticancer drug developer that has received investments from global pharmaceutical companies, including BMS. Its founder and CEO Jay Mei, has extensive experience in the field, working at the National Cancer Institute as well as various global pharmaceutical companies including Johnson & Johnson, Novartis, and Celgene, where he led global clinical trial programs. Based on this experience, the CEO founded Antengene, with its key focus of interest in blood cancer. Antengene is one of the few Chinese biotechs that have entered Korea. The company decided to enter Korea as it considers the country an important base in the Asia-Pacific region. The company is also actively engaged in open innovation with bio companies in Korea. It is currently conducting joint research with LegoChem Bio. Through such efforts, Antengene aims to address the unmet medical needs in the Asia-Pacific region, and further expand into a global company. At a virtual interview with Dailypharm on the 14th, CEO Mei (57) said, “We had decided early on that we would need to enter Korea as the country owns a top-class healthcare system, a solid infrastructure for clinical trials, and has a good environment for R&D. We plan to continue expanding our business through joint research in partnership with various Korean companies including Lego ChemBio.” The following is the QA with Antengene’s CEO Jay Mei. Jay Mei, CEO of Antengene-You chose selinexor, an oral anticancer drug you brought in from Karyopharm as the first product for commercialization in Korea. What do you think of selinexor’s vision? =Selinexor was approved as a treatment for multiple myeloma (MM) and diffuse large B-cell lymphoma (DLBCL) treatment by the FDA in July 2019. The drug has been also approved for the two indications in Korea in July last year. Selinexor is an oral selective inhibitor of nuclear export (SINE) that can be used in combination and as monotherapy. We are conducting trials in other blood cancers such as myelofibrosis and acute myeloid leukemia, and are investigating its use in T cell or NK cell-related lymphoma as well. WE plan to continue expanding selinexor’s indication in consideration of its scalability. -What is your key platform technology and pipeline? =Antengene has opted for a two-track approach in which the company seeks growth through partnership-based technology introductions and the development of original pipelines. In addition to Karyopharm, we have made partnership agreements with AstraZeneca, Celgene, and LegoChem Bio. For individual development, our scientists are developing new drugs by investigating new targets. We have a total of 15 progress in progress ranging from non-clinical trials to Phase III trials that are investigating small-molecule drugs, monoclonal antibody therapies, bispecific antibody drugs, ADCs, etc. The trials are underway in Asia and the United States, as well as in Japan and Europe. -What other product do you have in plan to commercialize following selinexor? =We are developing a new drug substance that has the same XP01 inhibiting mechanism of action as selinexor. We are conducting various clinical trials with Karyopharm for the drug, eltanexor (code name ATG016). The target indication is the high-risk group with myelodysplastic syndrome, and a global clinical trial currently in progress is a pivotal clinical trial prepared to be used as the basis for approval reviews. Also, Antengene is developing 6 other new drug candidates for commercialization. First, ‘ATG008' is a new drug candidate that can be used with mTOR inhibitors for the treatment of cervical cancer. The company plans to conduct a global trial if the ongoing clinical trial brings positive results. Also, a clinical trial for a PD-1-based bispecific antibody ‘ATG101' is underway in Australia for patients who cannot see a further effect from existing PD-(L) 1-based immunotherapies. Within the company, ATG101 is considered a unique substance that has the potential to become a ‘best-in-class’ drug. -What is the background on your partnership with LegoChem Bio in Korea? What is your prospect for ADC treatments? = 'ATG022' that we have in our pipeline is an ADC-based treatment that targets claudin-1. Claudin is quite often found in gastric cancer. Antengene is deeply interested in cancers with high prevalence in Asia like gastric cancer and has taken an interest in next-generation ADCs while developing ADCs. In our search for companies with new innovative technology for developing anticancer substances to conjugate with ADC platforms such as linkers or payloads, we came across LegoChem Bio. We believe LegoChem Bio owns a unique technology and strong potential for next-gen ADC development. Both companies have been searching intently for a new candidate substance after signing the partnership. -In addition to Antengene, the global entry of China-based biotechs and their collaboration with big pharmas have been increasing recently. What do you make of this trend? = Despite the remarkable economic development the region had made over the past 30 years, the unmet medical need in the Asian region is still relatively high. Just in the fields of multiple myeloma and lymphoma that Antengene frequently monitors, only half of the drugs approved in the Western countries are approved in Asia. As such, there are still many areas where patient accessibility needs to be increased. Thanks to the development of the economy and healthcare systems, the talent pool is rapidly increasing in Asia as well. As more and more Asian talents with graduate degrees or higher in biology, chemistry, and medicine enter society, there is now an abundant opportunity for Asia-based biopharmaceutical companies to utilize these talents. We believe that these environmental changes played a part in increasing collaboration opportunities for Asian-based biotech companies that have not entered the global market before. The entry of these companies will provide benefit the global patients by developing new drugs based on new technologies and medical knowledge. If European and American companies had fared well in the past 50 or 60 years, I think it is not time for Asian-based companies, including Korea, to play this role now. I think now is the right time to go global. -Many Korean biotechs are also seeking to enter the global market. However, the companies have trouble communicating with the FDA and designing the clinical trial protocols. As a biotech that has commercialized various products, what know-how do you have to share with the Korean companies? =To become a successful bio-company, the company should first own a competitive product or candidate substance. Next is talent. The company needs to recruit a lot of talented people who have global vision and experience, a deep cultural understanding of various countries and can work smoothly as a team with people from other cultures. In the case of our company, we had set the global strategy to expand into Asian countries including Korea, then to go global from the early stages of establishment. So we thought securing the two factors mentioned above was of utmost importance. In particular, as pharmaceuticals are one of the most highly regulated industries, collaboration with regulatory authorities is very important. Also, we have put in a lot of effort to secure a talented workforce that owns such job competencies. Also, finding a reliable local partner can be helpful if you don’t have enough time to set up a solid team in each market. Finding a good partner is as important as securing good talent in-house. This is why we have established partnerships with various companies including Karyopharm, AstraZeneca, Celgene, and LegoChem Bio. -Your Korean branch celebrated its 1st anniversary this year. What other activities do you have in plan for the Korean branch as well as other countries? = Antengene has established subsidiaries in Korea, China, Australia, Singapore, Hong Kong, Taiwan, and the United States, and is aiming to continue expanding in the future in terms of expanding pipelines and talent pools in addition to geographic areas. Currently, Antengene is positioning itself as an 'Asia+' company, and our urgent mission is to meet the unmet medical needs in many Asian countries. Selinexor has been approved in for this purpose in Korea, Australia, Singapore, and China, and is scheduled to be approved in Taiwan and Hong Kong within the year. The second step is to broaden the partnership with the self-developed substances to advance and become a truly global company. I first visited Korea while serving as head of a global clinical program at Novartis, and was also able to broaden my business understanding of Asian counties at Celgene. Building on my experiences, I am determined to drive Antengene's strong growth and expand its businesses in Asia.
- InterView
- Era of ₩30 bil novel homegrown drug Suganon begins now
- by Feb 03, 2022 05:57am
- Annual sales of the 26th homegrown new drug ‘Suganon (evogliptin)’ family that was developed by Dong-A ST has reached ₩30 billion 5 years into its release. During an interview with Dailypharm, Sung-Woo Lee, the GPM who oversees the marketing of Suganon, said, “Market share of Suganon has been steadily increasing due to its superior efficacy data, such as its strong effect in lowering blood sugar levels and its stable maintenance of glycemic variability, as well as efforts to improve convenience in intake. With our product line-up, expanded indication, and active efforts to expand to the overseas market, we will continue to grow our homegrown new drug.” ◆Last latecomer Suganon makes a winning bid with ‘evidence’ Sungwoo Lee, Suganon GPM at Dong-A ST The DPP-4 inhibitor Suganon had not always been such a success. When the drug was released in March 2016, the DPP-4 inhibitor market was full of fierce competition with products from both multinational and domestic companies. MSD’s Januvia had been raising ₩140 billion in outpatient prescriptions in collaboration with Chong Kun Dang. Trajenta, which has been copromoted by Boehringer Ingelheim and Yuhan Corp had also raised ₩100 billion, and LG Chem’s individually developed Zemiglo raised ₩50 billion. In other words, it was already a difficult environment for a latecomer drug like Suganon to penetrate. After GPM Lee took charge of marketing Suganon in 2019, he had earnestly sought measures to differentiate the product from the other products. As a result, Suganon’s stronger blood sugar level lowering effect among DPP-4 inhibitors was emphasized and reaffirmed through the EVERGREEN clinical trial. Lee said, “Suganon’s sales increased after the drug’s differentiated effect was published in the SCI paper ‘DOM,’ in which the drug demonstrated a strong blood sugar level lowering effect and evidence with a new index, glycemic variability. Last year, the combination drug ‘Sugamet’ that contains both Suganon and metformin drove Suganon’s sales growth. Dong-A ST used metformin to reduce the tablet size of Sugamet twice. Last year, Sugamet recorded the highest growth among the 21 DPP-4 inhibitor products, with a 39.7% growth in sales. Lee said, “Suganon’s market share, which was the lowest among 9 DPP-4 inhibitors by 2018, rose to exceed 5% for the first time last year due to accumulated clinical data and its improved convenience in administration. ◆Suagnon increases domestic market share and heads out globally However, the company is not stopping there and is aiming higher. First of all, the company is expanding the Suganon lineup in line with the current trend in diabetes treatment. The new combination Dong-A ST had been working on since 3 years ago is a combination of Suganon and an SGLT-2 inhibitor ‘dapagliflozin (product name: Forxiga).’ With the need to introduce a new combination drug, the company had started clinical trials in full scale. Among DPP-4+SGLT-2 combination drugs, Boehringer Ingelheim’s ‘Esglito,’ MSD’s ‘Stegluzan,’ AstraZeneca’s ‘Qtern’ were approved, but none are being prescribed in earnest yet due to reimbursement issues. However, as the insurance authorities are discussing accepting the ‘class effect’ of SGLT-2 inhibitor combinations, the industry is looking forward to future changes in the environment. Dong-A ST is also in the midst of clinical for its DPP-4+SGLT-2 combination with the goal of launching the drug in the second half of next year. Dong-A ST has also started a global clinical trial for the calcific aortic valve disease indication. Dong-A ST will be conducting a Phase II/III trial led by Rednvia, which was jointly established by Dong-A ST and Bionvia. Lee explained, “We had confirmed Suganon’s strong effect in calcific aortic valve disease in animal models in the Phase II trial, and started Phase II/III clinical trials at various medical institutions including the Mayo clinic that will be completed by 2025. As 3% to 25% of patients over 65 are estimated to have the condition in North America, we believed it has high marketability for the indication there.” The Suganon family is recording active sales overseas as well. Starting in Indica in 2019, Suganon was also released in Russia, Brazil, and Argentina. The company is preparing to release Suganon in many other Latin American countries this year.
- InterView
- There shouldn't be any patients who can't receive new drugs
- by Eo, Yun-Ho Jan 05, 2022 05:59am
- Global pharmaceutical companies, which can be said to be the mainstay of the supply of new drugs. The KRPIA, which represents these companies, is also raising expectations in 2022. Multinational pharmaceutical companies' attention is more focused on the "appropriate value of new drugs" than ever before. With the advent of the so-called "high-priced drug era," it is becoming increasingly difficult to find a point of contact between the government and the pharmaceutical industry. There are still many discussions and suggestions regarding the drug price system, such as expanding the scope of application of the PE system, introducing pre-registration & post-evaluation, and adding drugs subject to negotiation and calculation. Dailypharm met with Lee Young-shin (64 yrs old), a full-time vice chairman of KRPIA, and heard about KRPIA's future plans for a period of expectation and transformation. -The regime will change in 2022. What is there to ask for a new government? Vice Chairman Lee Youngshin = In the new government, it is hoped that patients will not be able to receive treatment or health insurance coverage for severe disease treatments due to low income. In other words, it is expected that policies will be prepared and implemented so that there are no patients who are alienated and discriminated against from necessary treatments due to income and disease. The government is expanding its role as a global leader and has announced several plans to become a biopharmaceutical industry powerhouse. This seems to have originated from the belief that economic scale, public awareness, and potential technology have aspects of advanced countries. In order to become a biopharmaceutical powerhouse, one must not stay in "vaccine sovereignty" but have "new drug sovereignty," and sovereignty is being created. From a global perspective, Eco-systems and infrastructure in the pharmaceutical industry should be established, and open innovation should be established with an open mind. - What is the current issue or vision that the KRPIA wants to focus on the most this year? = In the new year, the KRPIA will also make efforts to help the pharmaceutical bio-industry take responsibility for public health and contribute to economic development. First of all, new drugs with new therapeutic paradigms such as state-of-the-art biopharmaceuticals are being released. We will actively participate in the improvement of policies and systems related to drugs and new drugs that allow domestic patients to access quickly, and promote the improvement of patients' access to new drugs as a top priority. - Then, what do you think was the biggest achievement of the KRPIA last year? = In the face of COVID-19 Pandemic, which is suffering worldwide, it was rewarding to secure the stability of the supply of imported medicines as a measure for the health rights of the people. The expected issues were identified in advance, discussions with regulators, and alternatives were presented, and continued to cooperate. As a result, it was able to receive active government support, such as replacing document screening of overseas surveys, utilizing online due diligence, allowing electronic documents, and activating counseling through video conferences, and stably supplying medicines without major problems. It is also proud that the COVID-19 vaccine developed by our member company contributed to the quarantine. What was the most regrettable issue as an association last year? = Last year, there was great progress and improvement in the regulatory environment to quickly develop and introduce COVID-19 vaccines and treatments, and we appreciate the government's efforts to do so. It is necessary for the government and industry to proactively prepare and discuss after post-Corona to prepare for the introduction of products due to the development of new technologies, patient access to new treatments, and electronic product manuals that can quickly provide the latest drug information. I hope there will be a lot of progress this year, and the association will continue to make efforts. - When talking about the contribution of multinational pharmaceutical companies to Korea, it is true that the investment of research funds in Korea was focused on phase 3 research. Is there a plan for basic research support? = According to the results of the annual R&D survey conducted by the Association on member companies, the growth rate of phase 1 and phase 2 corresponding to the initial clinical trial has recently increased significantly compared to the growth rate of phase 3. I know that many member companies are actively cooperating in various forms with domestic research institutes, schools, and bio-ventures to support basic research. However, since basic research is in the very early stages of research and development, the contents and progress cannot be disclosed.
- InterView
- Ibrance can be used regardless of underlying condition in BC
- by Dec 24, 2021 05:48am
- It has been 5 years since Pfizer’s breast cancer treatment ‘Ibrance (Palbociclib)’ was introduced to the Korean market. As the first cyclin-dependent kinase 4/6 (CDF 4/6) inhibitor, the drug had innovated the treatment paradigm for patients with metastatic and recurrent hormone receptor-positive (HR-positive) and human epidermal growth factor receptor 2-negative (HER2-negative) breast cancer. With 5 years' worth of accumulated data, Ibrance has settled in as a trusted and reliable drug for doctors. In particular, Ibrance is considered the ‘go-to drug’ for those who have underlying conditions or have side effect concerns. At a meeting with Dailypharm, Kyong-Hwa Park, Professor of Oncology and Hematology at Korea University Medical Center, said that “Ibrance is suitable for use in patients who have poor liver conditions, the elderly, and those with poor kidney functions as it demonstrated long-lasting effect with little side effects in the field. Detailed QA of Dailypharm’s interview with Professor Park is listed below. Professor Kyong-Hwa Park -What has the diagnostic status been like for breast cancer in Korea recently? =The number of breast cancer patients has increased greatly recently. Korea’s prevalence rate of breast cancer had surpassed Japan and is now ranked highest in Asia. One silver lining is that the long-term survival rate of Korean patients is very good. Although there are some drugs that are not reimbursed, Korea’s breast cancer treatment environment in terms of treatment, medical service, and accessibility is quite good. - After Ibrance, other CDK4/6 inhibitors have also been started to enter the market. Nevertheless, Ibrance seems to be considered as the drug that can be stably used due to the immense amount of accumulated data and prescription experience. = That is true. Ibrance is the first CDK4/6 inhibitor that was approved in Korea and owns the most amount of long-term data as well as clinical experience. This is why it is a good choice for patients with various concerns. For example, patients who are at risk of side effects due to underlying diseases, old age, etc, may require a period of adaptation when using a drug or take various medications. It would be difficult for the doctor to opt for other CDK4/6 inhibitors in these cases. - Ibrance demonstrated consistent efficacy in patients with underlying diseases in clinical trials. Were you also able to observe this in the field? = A more diverse range of patients always exist in the real world. Some patients are very old, there are those who have poor kidney function, those with bad heart function, and even liver cirrhosis. The number of patients with such underlying conditions increase immensely if we add those who have diabetes or high blood pressure. All of these patients may use Ibrance. For example, I have a patient who has rheumatoid arthritis. She had a fatty liver due to long-term use of rheumatoid arthritis drugs. By using Ibrance with liver condition management, the patient is currently on Ibrance for 4 years with stable liver function. The drug can also be stably used in patients with bad kidney function as well. Also, in Korea, there are patients who have bad liver due to hepatitis B or C. In these patients, we first use the drug and then adjust the dose if they develop leukopenia/neutropenia or thrombocytopenia. - A total of 3 CDK4/6 inhibitors including Verzenio, Kisqali, and Ibrance are available in the market. What other considerations do you make other than the patient’s underlying condition when selecting the kind of CDK 4/6 inhibitor for use? = ECG monitoring is required for the use of Kisqali, at least up to its second cycle, due to its influence on heart activity. Also, the drug may not be used in patients with observed QT prolongation. However, Kisqali is the only drug reimbursed for premenopausal patients who have never received endocrine therapy in Korea, and we induce menopause in such patients to allow the use of various drugs with the same indication. In the case of Verzenio, patients adapt quickly to the drug if educated well on the treatment process, but it is difficult to use in patients who may not be able to tolerate diarrhea. On the other hand, the advantage of Verzenio is that it is good for patients who have metastases to the liver or those who we would have considered using chemotherapy first in the past. -A large-scale real-world data on Ibrance was presented this year in the U.S. The study demonstrated PFS and OS improvement in combination with letrozole in the first-line. This may be similar or different in Korea’s case. How did you interpret the data? =The average age of breast cancer patients in the US is around 15 years older than those in Korea. Also, medical accessibly is not as good in the US as in Korea. However still, the real-world results were comparable to that of clinical trials. The median OS had not been reached yet, but I believe the results would show an improvement. With the younger patient population, better accessibility to treatment, and higher self-management ability, results in the Korean patient population in clinical trials has always exceeded the performance observed in the overall patient population. Therefore, I believe the real-world data in Korea would also come out similarly. -The role of CDK4/6 inhibitors is expected to continue to grow in the field of breast cancer treatment. What direction should Ibrance pursue in the aspect? =Ibrance is being frequently selected as a combination therapy option in novel endocrine therapy combination studies. Although it is currently used in combination with an aromatase inhibitor or faslodex in the first-line, many other 3rd generation oral endocrine therapies are also currently in development. And all of these oral therapies are being developed in combination with Ibrance, so I believe Ibrance will be able to solidify its position as a first-line treatment while switching its partner drugs. Also, PIK3CA mutation is a very important mechanism in endocrine resistance, and studies to tackle this with a three-drug combination are also being conducted using Ibrance. The toxicity of the three drugs does not overlap, so I believe it can be well used in this aspect as well.
- InterView
- KYMRIAH, a good but expensive medicine
- by Eo, Yun-Ho Nov 04, 2021 05:56am
- The cost of a single injection is 500 million won, but the era has come when cancer can be expected to be cured with that "once." Ultra-high-priced, high-tech new drugs are already approaching us. Kymriah (Tisagencleucel), a CAR-T treatment called dream anticancer, is the new drug. Kymriah obtained approval from the MFDS in March as the first treatment of the Advanced Regenerative Bio Act. Kymriah dramatically improved the survival period of patients with recurrent and refractory end-stage blood cancer, who no longer had treatment options available with a single treatment, and even confirmed the possibility of long-term survival. ◆The essence of process-customized treatment required for each patient Kymriah is an innovative personalized one-shot treatment that utilizes the patient's immune cells, and is an anticancer drug with all the characteristics of cell, gene, and immune treatments. As it is a non-reimbursed treatment, it is different from previous treatments from the mechanism and manufacturing process of manufacturing. First, the patient's immune cells are extracted. Afterwards, a receptor that recognizes cancer cells is inserted into the cell surface to form a cell with strong power, that is, a chimera antigen receptor, and injected into the patient. Kymriah's indication is ▲the treatment of adult patients with recurrent or refractory diffuse large-B-cell lymphoma (DLBCL) after two or more systemic treatments and ▲ the treatment of post-transplantation recurrence or secondary recurrence and subsequent recurrence or refractory B-cell acute lymphocytic leukemia (B-ALL, B-Acute Lymphoblastic leukemia) in children and young adult patients under the age of 25. The number of DLBCL and B-ALL patients who refused or recurred to existing treatment was about 200 in Korea, and until Kymriah's approval, there were no alternative treatment options or standard treatment was not established, so life expectancy was only 6 months. In fact, the median survival period of DLBCL patients who failed secondary treatment in Korea is around 4.73 months, and about 70% of patients who failed secondary treatment repeatedly perform rescue chemotherapy. The Kymriah permit presented patients who were no longer in need of treatment with another hope for long-term survival. According to clinical trials in patients with recurrent or refractory DLBCL, the overall response rate in patients administered Kymriah was 53%, of which 39.1% reached complete remission. In addition, in the case of clinical trials in patients with recurrent or refractory B-ALL, 8 out of 10 patients (82%) reached complete response (CR), and 98% of patients who reached remission were negative. ◆ In Korea, more than 5 cases were administered. Kymriah Center was established Kymriah is still a non-reimbursed drug and it is difficult to say that sales have been activated in Korea. However, preparation work for prescription is active. In particular, Big 5 advanced general hospitals are moving rapidly. According to related industries, Big 5 general hospitals, including Seoul National University Hospital, AMC, Seoul St. Mary's Hospital, and Sinchon Severance Hospital, are undergoing management approval procedures such as human cells, and Samsung Medical Center has already completed approval. Among them, in the case of Seoul National University Hospital, Kymriah (Tisagenlecleucel) passed the Drug Commission (DC) in April, and Samsung Medical Center also began prescriptions in May. Novartis, a developer of Kymriah, allows to pay by establishing a general hospital and Kymriah center. Kymriah Center will open in May at Samsung Medical Center and Seoul National University Hospital, respectively, and the rest of the upper-level hospitals are expected to join the center later. In order to establish a center, hospitals must obtain permission for management businesses such as human cells under the newly established Advanced Regeneration Bio Act, which means that medical institutions are actively working on preparing all matters. according to the Dailypharm, more than five Kymriah doses have already been made, even though they are non-reimbursed, and 10 prescriptions will be made within this year. The era of CAR-T is certainly beginning. ◆The insurance benefit registration procedure is also an issue Kymriah's insurance benefit registration is also an issue. It is unusual for interest in one drug to increase to this extent because it is such an expensive drug and there are so many patients waiting. In March, the drug was approved by the MFDS using the Drug Approval-Benefit Linkage System. And it was first introduced to The HIRA's Cancer Drugs Benefit Appraisal Committee in September, but was put on hold. When the results were released through the media, the leukemia association criticized the government and pharmaceutical companies in a statement. The patient association earlier criticized Kymriah for the delay in the proposal of the Cancer Drugs Benefit Appraisal Committee. Eventually, Kymriah passed the Cancer Drugs Benefit Appraisal Committee in October. It is encouraging to pass this deliberation, but there is a high possibility that there was a burden as it was so focused on attention. Novartis' Korea subsidiary's plan to share finances and its willingness to persuade its headquarters are expected to be key. Kymriah, a drug that is too good but too expensive, is just the beginning.
- InterView
- Patients with severe diseases don't really like Mooncare
- by Lee, Jeong-Hwan Oct 28, 2021 05:59am
- Health insurance authorities should recognize that as universal health and welfare increases the coverage of mild diseases, the access to drugs for severely rare and intractable diseases is greatly reduced. It is nonsense that policies to strengthen coverage such as herbal medicine benefits are implemented without economic evaluation today, when there are many serious drugs that cannot be reimbursed due to the adequacy of benefits." This year's parliamentary audit of the National Assembly's Health and Welfare Committee also dealt with the issue of accessibility to patients with ultra-high-priced one-shot treatments, which cost hundreds of millions of won per dose. Technologies for treating severe rare and intractable diseases such as cancer and autoimmune diseases are rapidly developing, but health insurance finances for benefit-applied drugs are not keeping up. On the 27th, Dailypharm met Professor Lee Hyung-ki (57), a professor of clinical pharmacology at Seoul National University Hospital, and asked about the direction of Korea's health insurance benefit system for expensive drugs. Professor Lee Hyung-ki said the so-called "Moon Jae In Care" implemented by the current government is not welcome at all to patients with severe rare and incurable diseases. While it is strict for drugs developed by global pharmaceutical companies or domestic pharmaceutical companies, it is unreasonable to enter the insurance right without any problems. Professor Lee said the government should adopt a selective differential welfare method. The basic direction for the government is to focus on expanding the benefits of ultra-high-priced treatments that are inaccessible even with average or high benefits, leaving treatments or diagnoses for mild diseases that can be spent on average annual income. According to a survey of 787 domestic companies by Job Korea, the annual salary of new college graduates this year is 41.21 million won for large companies and 27.93 million won for small and medium-sized companies. According to the analysis of business reports by each company of the top 100 companies in market capitalization, the average annual salary of employees of large companies is 83.22 million won. In addition, this year's average annual salary for small and medium-sized workers is 28 million won for employees and 57 million won for managers. Professor Lee points out that it takes about two years for one new drug to enter the benefit range, although the government claims to be completing the benefits evaluation about 300 days. Professor Lee said, "In the case of anticancer drugs, it takes about two years to receive health insurance benefits. In this case, pharmaceutical companies that have developed treatments and patients waiting for insurance will suffer from double regulations, he explained. Professor Lee added, "Since we mainly focus on price control, it becomes difficult to be reimbursed and patient difficulties increase." Asked by a reporter if it would be difficult to apply new drug insurance benefits that did not take into account health insurance finances at all, Professor Lee said, "There are too many policies such as herbal medicine benefits to put health insurance finances as a justification." Professor Lee criticizes that health insurance benefits are often made without standards or principles in areas that are difficult to accept from the perspective of new drug development pharmaceutical companies and severely ill patients. Professor Lee said, "The ultra-high-priced drug itself is, in the end, an innovative drug. Patients who have been confirmed to be effective will have an experience of changing their lives and not losing their lives. "Kymriah treatment costs hundreds of millions of won. It is a level that individual patients cannot bear, he pointed out. Professor Lee said, "The national health insurance funding alone will inevitably reach the limit of the medical insurance drug cost support system." Professor Lee said, "It is necessary to consider raising a third fund and financial source. The government should show leadership to expand fiscal sources, he said. Professor Lee also criticized the lack of economic evaluation tools for ultra-high-priced one-shot treatments. There is no additional system other than RSA, and ICER are too low. Professor Lee said, "I understand that the benefit evaluation tool has become more flexible than before. The problem is that health insurance authorities rely only on ICER. In particular, if the standard treatment is already expensive, no matter how innovative the new drug is, accessibility will not increase, he stressed.
- InterView
- Merck will “focus on specialty care capabilities"
- by Eo, Yun-Ho Oct 21, 2021 05:14am
- Javed Alam, General Manager of Merck Biopharma Korea The global chemical and pharmaceutical company Merck is working intently to strengthen its capabilities in the pharmaceutical sector. The company, whose main areas of expertise are liquid crystal and LED, has also been continuing its commitment to introduce new drugs, starting with the anticancer drug ‘Erbitux (cetuximanb),’ the immune-oncology PD-L1 inhibitor ‘Bavencio (avelumab)’ that was co-developed with Pfizer, to the recently approved multiple sclerosis treatment 'Mavenclad (cladribine).’ Dailypharm met with Javed Alam, the General Manager of Merck Biopharma Korea, who has celebrated his 2nd year at the company, to hear about Merck’s vision in the healthcare business. -You have spent most of your time here since your appointment in the COVID-19 pandemic. Could you tell us about your impressions and thoughts about your work here in Korea, and the achievements that were made?. I would have to say my time here was crisis after crisis due to the COVID-19 pandemic. However, despite the COVID-19 pandemic, we were able to protect our employee’s health and safety while continuing communication with our customers and ensuring a smooth supply of products for our patients, based on which we achieved business growth during the past 2 years. In the midst of the COVID-19 crisis, our company had focused on Specialty Care and efficiently reorganized the organization, and carried out our roles smoothly so that there were no disruptions in the supply of our products. In addition, we made various efforts such as actively embracing the use of digital channels to communicate with our customers to frequently update them on the company’s situation. Also, I take special pride in the fact that no Merck Biopharma Korea employee has had COVID-19. -- New changes have occurred in the Pharma business area, such as the launch of new products. Does this mean the company is seeking a change of tone? Merck’s goal in global healthcare is to become a Global Specialty Innovator, under which Merck Biopharma Korea aims to become the most innovative specialty care company in Korea. To achieve this goal, the company had to undergo various changes in its business model, company structure, organization, etc. While making such changes, we also changed our corporate culture to focus on restructuring the business model to focus on specialty care and digitalization. Through these efforts, we expanded our digital channel from 1 to 8, including webinars, websites, and web meetings. Also, the company made various changes structure-wise, and these various efforts have fortunately paid off to benefit the company. -I can see the company’s strong commitment to specialty care. The Specialty Care unit covers rare and incurable diseases that are difficult to diagnose or cure. The number of patients and specialists for such diseases is relatively small, and that is why it is also an area that has the highest unmet need from patients. Among various areas of specialty care, Merck is focusing on 4 areas - Immuno-oncology∙Oncology, Neurology∙Immunology, Infertility, and Endocrinology. Erbitux has been showing good performance in our existing Immuno-oncology unit. In the recent KSMO Annual Meeting, & Conference we have presented the results of OPTIM1SE that demonstrated the drug’s clinical safety and efficacy in Korean patients. In addition, we have also released the immuno-oncology drug ‘Bavencio (avelumab)’ that was co-developed with Pfizer in our efforts to become a specialty care leader. Bavencio is a treatment for Merkel cell carcinoma (MCC) which is a rare and aggressive type of cancer that has a 5-year survival rate of 0-20%. I believe we were able to provide new hope to these patients with Bavencio. In Neurology∙Immunology, we have the multiple sclerosis treatment Mavenclad (cladribine). Multiple sclerosis occurs most commonly in women in their prime - 20s to 30s - causing difficulty in their life and treatment. However, Mavenclad’s innovative dosing and administration allows patients to only take Mavenclad for up to 20 days over 2 years, and then be free from additional dosing requirements for the other 2 years. -What kind of efforts have Merck Biopharma Korea made to advance Korea’s healthcare industry? We have been actively participating in clinical programs and global trials to increase Korea’s level of contribution and influence. Korea is involved in all of the 20 global clinical programs at Merck including its key 7 programs. By actively participating in R&D and the clinical stage, we are increasing our contribution to Merck’s portfolio and ultimately increasing Korea’s influence in the global market. Also, we have been making efforts to increase Korea’s contribution in earlier stage innovation. For example, we help promising startup venture companies in Korea to connect with Merck’s innovation program. The ‘Merck Accelerator program’ has supported a total of 12 companies worldwide until now, and ‘Inhand Plus,’ a Korean startup, was the first in Korea to be selected to receive benefits from the program. -Could you briefly introduce the company’s pipeline products that you plan to introduce in Korea in the future? Merck Biopharma Korea is striving to showcase innovation in all areas of its involvement, therefore, you can continue to expect innovative changes and products from us in the future. In the past, the company had depended heavily on primary care products. However, now, as the Global Specialty Innovator, we hope to become an unrivaled company in specialty areas. Our first goal is to maximize our Korean patients’ accessibility to the company’s excellent pipeline products. Merck Global Is focusing on enriching its entire pipeline, and we will continue our efforts to promptly and broadly introduce all the innovative solutions, products, and technology that is and will be developed by Merck to our patients in Korea.
- InterView
- "A tenure professor’s calling is in developing a new drug"
- by Sep 17, 2021 05:56am
- On August 24th, the Hemato-Oncology Department of the Eijeongbu Eulji Medical Center was busy preparing for its new occupant. Professor Dongwook Kim (60), who looked new to his office, was busy discussing matters with various visitors including the hospital employees. Although the center had opened less than 6 months ago, its Hematol-Oncology Department looked more vibrant than ever. Professor Dongwook Kim, one of the leading authorities in the field of Chronic Myelogenous Leukemia (CML), had joined Eijeongbu Eulji Medical Center of the Eulji University after serving 30 years at the Seoul St.Mary’s Hospital. During his term at Seoul St.Mary’s Hospital, Professor Kim had made many first-ever achievements. At St.Mary's Kim had led the research of the first targeted therapy, ‘Gleevec,’ and many other next-generation drugs, and had also led the study on ‘Supect,’ the only locally developed targeted therapy for CML. His focus on gene analysis, to identify the causes why patients show different treatment effects, had laid the grounds for Korea's treatment environment to advance into precision medicine. Kim's efforts paid off, and the Seoul St.Mary’s Hospital became the first hospital to establish a center specializing in blood disorders - the Catholic Hematology Hospital - at which Kim served as the founding director. Professor Dongwook Kim When asked about why he joined Eijeongbu Eulji Medical Center after achieving so much, Kim's answer was “to conduct more research for a longer period of time.” Kim talked about a professor he met at the International Society of Hematology conference. The professor, who was over 90, came to chair one of the discussion sessions with a cane in one hand. This had left a deep impression in Kim’s mind. Also, he said that there are professors over the age of 80 in the Leukemia Network, for which 35 experts around the globe meet every 5 years to establish the standard of care for CML. Unfortunately, the circumstances at St.Mary’s Hospital were unfit for a professor to continue researching for the rest of his life. In medical schools in Korea, professors generally retire from his/her university at age 65, and stays as a professor emeritus, then continues work at a different hospital for 5 years before retiring. “As a director, I’ve watched many of my seniors retire. These able professors perform surgeries until the last day of their retirement. After 55 years of age, most professors experience reduced consultation hours and a lack of research labs. I also experienced this. My lab also saw a decline in funding and was on the verge of reducing our staff. In Korea, the government does not give government projects to professors over the age of 60. On the contrary, many senior professors over the age of 80 and even 90 take on research projects in Europe and the U.S. It is that different." With only 5 years left to retirement, Kim was also concerned about his patients, as they require lifelong treatment. Many patients asked professor Kim about his retirement plans. Those that were recently diagnosed showed the most concern. That was why Professor Kim started to consider seeking an environment where he can continue on his research without worries about retirement or lab reductions. And Eijeongbu Eulji Medical Center was the perfect place for Kim. “The Eijeongbu Eulji Medical Center promised full support and a stable environment where I could conduct research with my researchers for a long period of time. Thanks to such support, we are currently setting our new lab in the new building behind. We plan to sign MOUs with KAIST, UNIST, and Kwangwoon University and conduct joint research after my lab officially opens.” Professor Kim said, “We have already been planning various research projects. One is ‘Investigation on the single-cell dynamics related to the occurrence/recurrence of CML,’ which was selected as a research project by the government last year. The project aims to investigate the cause of CML and why the treatment effect differs in each patient." In other words, Kim and his team will attempt to find the cause of different treatment effects by analyzing the patient’s genes and the tens of thousands of cells in their blood. If the team discovers a gene related to leukemia, this may enable personalized treatment for each patient. This is what Professor Kim is investing most efforts in. Also, Kim is actively participating in the development of new drugs like ‘Supect’ in collaboration with bio ventures. Also, he had joined in the development of AI that can recommend appropriate treatment for each patient since 2 years ago. Kim plans to complete the government project within 5 years. Kim believes that the research will enable HCPs to discern which treatment is required for each patient according to their genes. Two new candidates were already discovered for new drug development. Also, the AI that Kim had started developing 2 years ago is now being tested in practice. After inserting all the characteristics of a patient from his/her age, gender, favorite food to genetic disorders, the program selects the most appropriate treatment among the 5 targeted therapies for CML, then recommends further measures according to each patient’s treatment response and side effects such as dose adjustments or discontinuation and switching. The goal is to be able to completely replace experts in the field. One of the questions I encounter most often during lectures is on ‘What drug to select.’ We aim to build an AI that can provide a perfect answer to that question. We are formulating the increase in speed, degree, grade of cancer cells to predict which patients will experience recurrence and how fast. This will allow us to predict how likely a patient may discontinue treatment within 5 years.“