- LOGIN
- MemberShip
- 2025-12-24 03:01:33
- Adenovirus for AZ vaccine, Sinopharm uses inactivated virus
- by Lee, Tak-Sun | translator Byun Kyung A | 2021-01-11 06:10:32
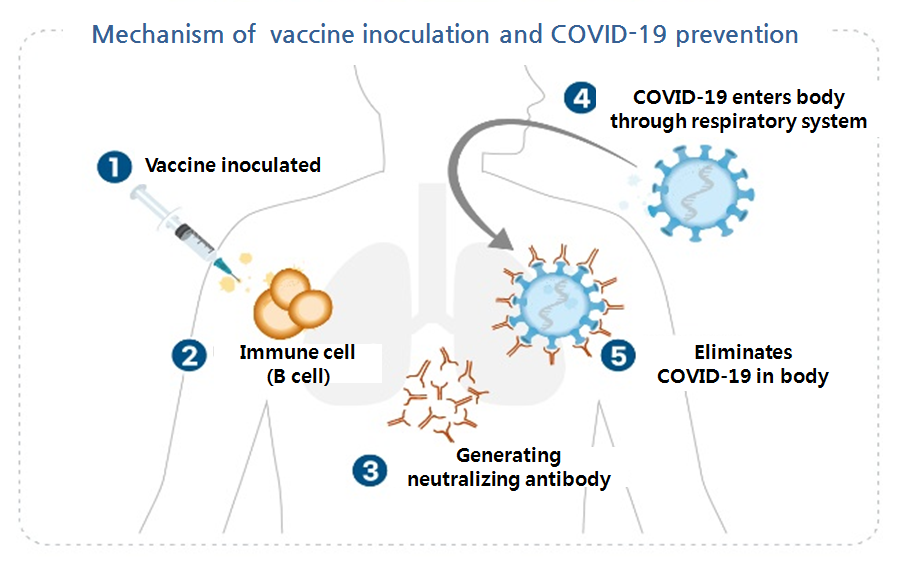
Diverse types of COVID-19 vaccines are currently in development.
Pfizer and Moderna’s vaccine candidates are based on RNA, when AstraZeneca’s is a virus vector and Korean-based SK Bioscience’ is a recombinant vaccine.
These types of vaccine have their respective strengths and weaknesses.
Some types have been commercialized already, but mRNA vaccine has never been commercialized, making the COVID-19 vaccine the first product.
On Jan.
7, South Korea’s Ministry of Food and Drug Safety (MFDS) explained about the different types of COVID-19 in development and their mechanisms.
The international effort to promptly seek COVID-19 vaccine has pushed pharmaceutical companies to explore a variety of vaccine platform technologies, including virus vector vaccine, mRNA vaccine, recombinant DNA vaccine and inactivated vaccine.
AstraZeneca and Janssen, already signed the procurement deal with South Korea, use the mechanism for their vaccines.
The AstraZeneca’s vaccine uses adenovirus that only infects chimpanzees.
Compared to mRNA vaccine, the virus vector vaccine is considered to be more stable around heat.
But the vaccine type requires cold chain maintained at 4 degrees Celsius as it uses a live adenovirus.
So far, Janssen’s Ebola vaccine using the mechanism was the only vaccine approved to market.
The U.K.
health authority has approved AstraZeneca’s vaccine as of Dec.
30, 2020 for an emergency use, whereas European Medicines Agency (EMA) is reportedly conducting an evaluation from October last year.
In South Korea, MFDS is currently reviewing the vaccine applied for approval on Jan.
4, 2021.
Janssen’s (Johnson and Johnson) investigational vaccine is conducting a Phase III clinical trial since September 2020.
The vaccine has already requested for evaluation in South Korea (Dec.
22, 2020) with the non-clinical and quality relevant evidences.
A Korean company Cellid’s candidate vaccine is in process of Phase 1/2 clinical study.
In development by Pfizer and Moderna, mRNA vaccine injects antigen gene as a messenger RNA form to generate antigen protein that instigates immune response.
The type of vaccine is to be introduced to South Korea later this year.
Although the particular vaccine type can be mass produced with fast manufacturing speed, maintaining the stability is extremely difficult with the RNA easily broken down by ribonuclease.
Accordingly, the vaccine requires cold chain with temperature kept at around minus 20 degrees Celsius or minus 75 degrees Celsius.
And it would be the first time for the type of vaccine to be commercialized.
RNA, or ribonucleic acid, is one of two nucleic acids in a cell, which carries genetic information and adjusts genetic expression.
Currently, the U.K (Dec.
2, 2020), the U.S.
(Dec.
11, 2020) and Canada (Dec.
9, 2020) have granted an emergency approval on Pfizer’s vaccine, whereas Switzerland (Dec.
19, 2020) and EU (Dec.
21, 2020) have conditionally approved the vaccine use.
MFDS elaborated the ministry is positive about the use of Pfizer’s vaccine as it is widely used all around the world, and even the World Health Organization (WHO) also granted approval on the emergency use (Dec.
31, 2020).
The vaccine has applied for evaluation on its non-clinical and clinical data on Dec.
18 last year.
Moderna’s vaccine has been approved for emergency use in the U.S.
(Dec.
18, 2020) and conditionally cleared by EU (Jan.
6, 2021).
The vaccine has not started the review and approval process in South Korea.
From South Korea, Genexine and Gene One Life Science are respectively developing DNA vaccine, similar to the mRNA vaccine.
DNA, or deoxyribonucleic acid, is one of two nucleic acids in a cell, which archives and conserves genetic information.
Novavax and SK working on recombinant vaccine already used for HBV and HPV vaccine Recombinant vaccine directly injects antigen protein made from recombinant DNA technology to induce immune response, which is one of the most commonly used vaccine platforms.
Because the recombinant antigen protein could be insufficient for the full immune response, generally an administration route with vaccine adjuvant is needed.
But it is known to be the highly safe vaccine for it has been used throughout the time.
Hepatitis B virus vaccine and HPV vaccine use the platform, for instance.
Novavax is using the platform for a COVID-19 vaccine with an ongoing Phase III clinical trial started from September last year.
Regardless, it has not been approved by any foreign health authority.
In South Korea, SK Bioscience is running a Phase 1/2 trial for the vaccine candidate.
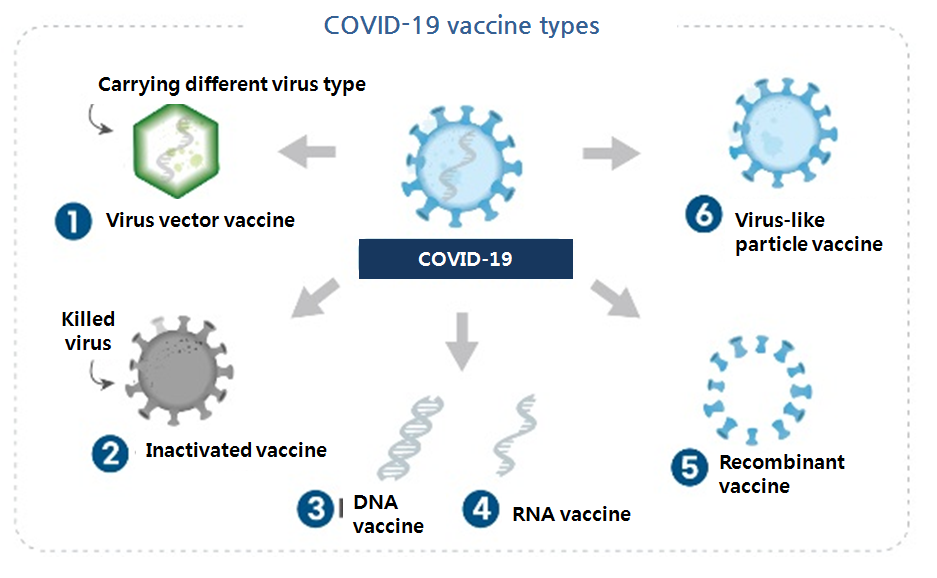
A number of vaccines are made with the technology.
It can be rapidly developed when the infectious virus is collected, and the manufacturing process is comparatively simple and it has an advantage of having outstanding neutralizing antibody.
However, for the COVID-19, the vaccine would require a manufacturing facility certified over Biosafety Level 3 (BL3).
Biosafety level indicates the level of a facility to safely handle potentially lethal infectious pathogen and depending on the level of the lethal disease, the facility is given a level ranging from BL1 to BL4.
Hepatitis A vaccine, injected polio vaccine and Japanese encephalitis vaccine use the inactivated virus platform.
A Chinese-based Sinopharm has developed COVID-19 vaccine with the technology green lit for use on July 22, 2020 in China.
And according to WHO’s COVID-19 vaccine candidate update report, CanSino Bio (virus vector vaccine) and Gamaleya (virus vector vaccine) are also working on other types of COVID-19 vaccines as well.
Considering the public’s heightened interest on COVID-19 vaccine, MFDS stated it would constantly update the vaccine’s efficacy and safety information, and focus on creating an environment for the people to get inoculated with no concern.
Moreover, the ministry official added they would do their best for the people to use the safe vaccine by strictly reviewing and managing safety and efficacy of the approved vaccine.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









