- LOGIN
- MemberShip
- 2025-12-24 11:44:44
- 74% relying on imported substance at risk amid COVID-19
- by Lee, Jeong-Hwan | translator Byun Kyung A | 2020-08-13 06:25:29

They advise the Korean government should set out a plan to promote diversification of overseas substance supplier and domestic manufacturing of essential substances, as well as to tighten safety management of the imported substances.
The experts also recommended the government to work on preventive measures as they had to revoke approved licenses on biopharmaceuticals like Kolon Life Science’ Invossa and Medytox’ Meditoxin.
On Aug.
10, the National Assembly Research Service published Health and Welfare Committee edition of ‘National Assembly Audit Issue Analysis 2020’ and gave advices to the government.
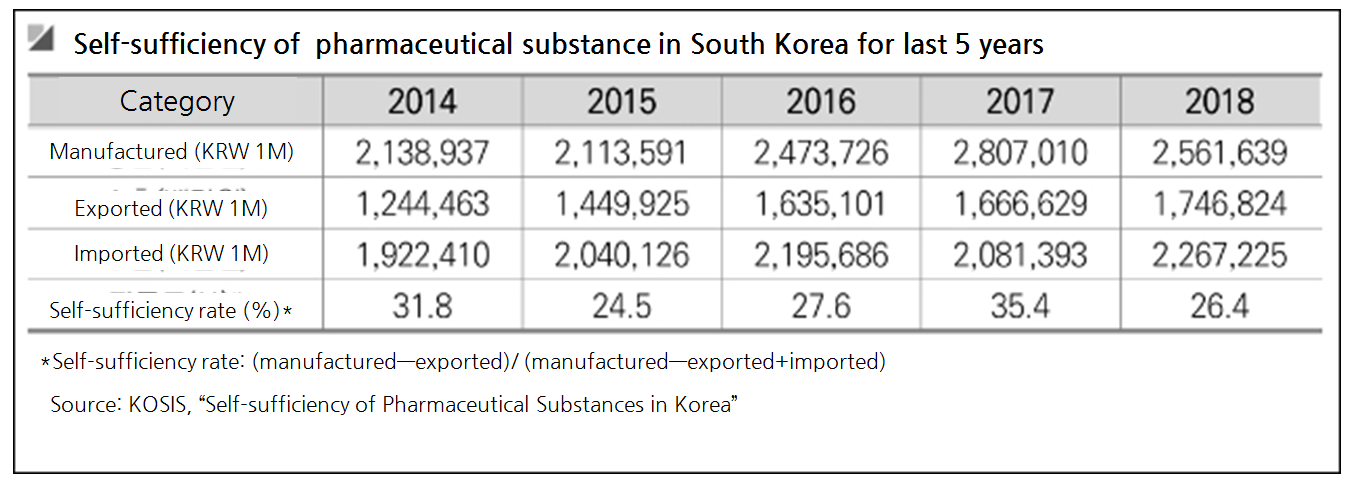
◆Dependency on imported pharmaceutical substance worsens: Pharmaceutical substances can be categorized either as an ‘active pharmaceutical ingredient (API)’ expressing the drug effect or an ‘intermediates’ essential for making APIs.
In last five years, Korea has been manufacturing 31.8 percent, 24.5 percent, 27.6 percent, 35.4 percent and 26.4 percent of pharmaceutical substances in year 2014 through 2018, respectively.
Korea’s pharmaceutical substance dependency on imported substances is as high as about 74 percent.
As of 2018, Korea has imported 33 percent of substances from China and 9.5 percent from India.
In fact, Korea Pharmaceutical and Bio-Pharma Manufacturers Association (KPBMA) conducted a survey in last February and reported Korean pharmaceutical and bio companies have stocks of pharmaceutical substances lasting two to four months.
In particular, the Research Service pointed out carcinogen contamination incidents occurred as the pharmaceutical companies neglected safety management in pharmaceutical substances although it can critically affect quality and safety in finished products.
In November 2019, Ministry of Food and Drug Safety (MFDS) has fully investigated valsartan, ranitidine and nizatidine when impurity in synthetic substances was discovered.
The National Assembly experts evaluate the pharmaceutical substance suppliers should be diversified and essential substances should be encouraged to be manufactured domestically.
And they also advised the Korean pharmaceutical industry to enhance safety measure in imported substances as the industry is heavily dependent on overseas suppliers.
The National Assembly Research Service recommended, “Some criticizes it is an excessive action to strengthen the management of substances imported from China and India, because of their low significance and risk.
But if the pandemic prolongs or another novel infectious disease breaks out, then the lack of good quality substance supply could delay production of finished products in Korea,” therefore, “The government should induce the industry to diversify overseas suppliers to maintain a good flow of supply and to manufacture essential substances.” The researchers added, “MFDS has disclosed plans to reinforce imported pharmaceutical substance safety control by introducing pre-registration system on overseas substance manufacturer to confirm Good Manufacturing Practice (GMP) compliance and to perform on-site investigation for quality control and management,” and “These pharmaceutical substances need more attention as the industry is relying heavily on India and China for importation of 715 cases (25.6 percent) and 227 cases (9.9 percent), respectively.” ◆Tightening pharmaceutical assessment procedure: The National Assembly Research Service has also urged the government to draw up a plan to prevent incidents like revoking the license on osteoarthritis gene therapy Invossa and botulinum toxin medicine Meditoxin for manufacturing and selling drugs different from the verified item.
The investigators not only reprimanded Kolon Life Science and Medytox for their unethical practices, but also criticized MFDS’ verification procedure.
Accordingly, MFDS presented their plan to strictly investigate and penalize such data manipulation cases with zero tolerance policy.
The researchers, however, pointed out the impact on patients’ safety should not be ignored regardless of MFDS stating the safety in Invossa and Meditoxin should not be a problem.
As a solution, the researchers recommended the government to tighten the GMP compliance management and adding more information on precautions when license revocation and recall recurs.
The National Assembly Research Service noted, “MFDS aims to conduct a randomized investigation even on drugs with first tier toxicity risk level to prevent data manipulation, and also raise the severity level of penalty on companies financially benefited from an item approved with data manipulation.”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









