- LOGIN
- MemberShip
- 2025-12-24 11:43:34
- Biosimilars are growing at 52% among pharmaceutical exports
- by Lee, Hye-Kyung | translator Choi HeeYoung | 2020-07-30 06:18:50
Pharmaceutical exports in the first half of this year were $3.8 billion, up 52.5% from the previous year's $2.5 billion.
Biosimilars account for 52% of export items ($1.98 billion).

The announcement was made by the director of the Health Industry Innovation Planning Team, Dong-Woo Han.
In particular, in the first half of this year, despite the deteriorating external conditions caused by the worldwide spread of COVID-19, the domestic health industry emerged as a growth export industry as it was rated as a 'K-defense' model country.
Since the occurrence of COVID-19, exports of diagnostic devices and sanitary products have surged, and domestic biopharmaceutical exports have continued to expand, ranking sixth in the export rankings, rising four levels compared to the same period last year.
◆Export trends in the first half of this year=exports of the health industry in the first half totaled $9.6 billion, followed by pharmaceutical products of $3.8 billion, cosmetics of $3.4 billion, and medical devices of $2.3 billion.
In the case of pharmaceuticals, exports of domestically produced disinfectants (Korea Customs Service's import and export classification HSK 3808940000) increased explosively ($3.35 million → $230 million) compared to the same period last year, and exports increased 52.5% year-on-year.
Exports to the United States, where COVID-19 is continuously spreading, surged since March, accounting for 52.1% of total disinfectant exports, followed by 25.6% in Japan and 5.4%.
in China.
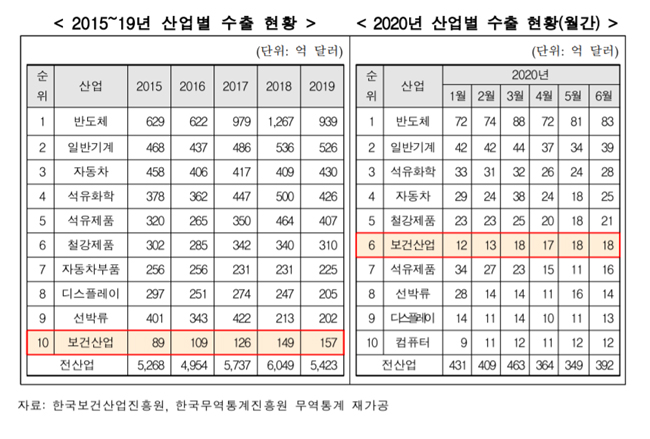
Exports of medical device diagnostic products have increased significantly since March, and have exported $730 million in the first half of the year to 173 countries such as the United States, Brazil, India, and Italy.
This is 31.4% of total exports.
However, despite increasing exports of diagnostic products, ultrasound imaging equipment (US$50.1 million → US$15 million) and implants (US$54.5 million → US$48.8 million), faced disruption in face-to-face operations in China, suspension of dental operations, and reduction in inpatients after COVID-19 For example, sluggish earnings also contributed to the decline in exports.
The total number of workers in the health industry in the first half of this year was 931,000, an increase of 29,000 compared to the same period last year.
By sector, drugs were 74,000, medical devices 52,000, cosmetics 37,000, and medical services 768,000.
In the field of pharmaceuticals, the number of workers increased 4.2% compared to the same period last year due to the development and investment of influenza vaccines caused by COVID-19.
Among the detailed industries, the number of drug manufacturing workers increased by 2,043 compared to the same period last year, the largest increase, and the manufacturing of pharmaceutical compounds and antibiotics increased by 533.
◆Management performance in the first quarter of this year=280 companies listed in the health industry, and the total sales amounted to ₩1.07 trillion, an increase of ₩1.1 trillion compared to the same period last year.
In terms of sales growth by sector, pharmaceuticals are 20.5%, medical devices 28.2%, and cosmetics 5.6%.
The R&D expenditure amounted to ₩800 billion, accounting for 41.5% of pharmaceuticals and 7.4% of medical devices.
156 companies were listed in the first quarter, with sales of ₩6 trillion.
Despite the COVID-19, the domestic prescription drug market rose slightly, and the sales of pharmaceutical companies increased due to increased sales of biosimilar products to the European and US markets.
(Inflectra, Truxima, Herzuma), and biosimilar products are continuing to be popular in Europe (Ramshima SC) and Chong Kun Dang's existing flagship items and the growth of newly introduced items (Kcab Prolia) has been strong.
Sales growth by pharmaceutical companies increased by 159.6% for Celltrion Healthcare, 80.5% for Celltrion, 25.2% for Chong Kun, 65.3% for Samsung Biologics, and 41.1% for Dong-A ST.
The KHIDI predicted that the domestic pharmaceutical industry will continue to maintain its competitive edge over global pharmaceutical companies by preoccupying the market by launching new products and expanding prescriptions for biosimilar products in Europe and the US.
◆Accelerated advancement into biosimilars =The creation of high value-added products through the expansion of global market share, such as the launch of new drugs in the global market and the expansion of overseas technology transfer, led to the export of medicines.
Among domestic pharmaceutical companies, Celltrion and Samsung Bioepis are preoccupying the market by obtaining first biosimilars in Europe and the United States.
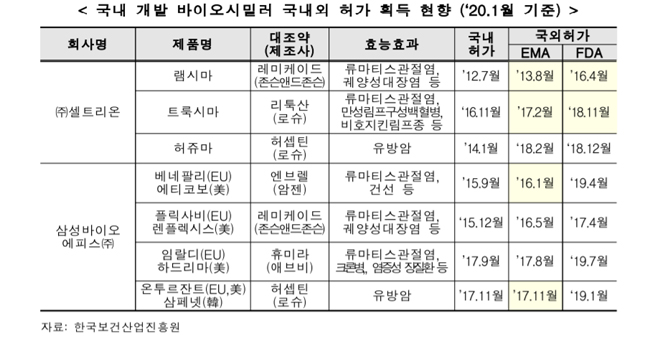
As of January of this year, the status of domestic and overseas biosimilar licenses obtained at domestic and international markets is as follows: Celltrion’s Remsima, Truxima, and Herzuma, Samsung Bioepis’ Benepali, Eticovo, Flixabi, Renflexis, Imraldi, Hadlima, Ontruzant, and Samfenet.
“The global economy and supply chain uncertainty have increased due to the prolonged COVID-19, but the domestic health industry has maintained growth compared to other industries, such as exports, jobs, and management performance, until the first half,” said Dong-Woo Han.
He emphasized, "In the future, the domestic health industry needs to support policy to preemptively prepare for the post COVID-19 era by accelerating the Korean version of the new deal, such as strengthening the D.N.A ecosystem and non-face-to-digital transformation."
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









