- LOGIN
- MemberShip
- 2025-12-24 17:52:14
- There are 17 cases of COVID-19 tx with stem cells worldwide
- by 박상준 | translator Choi HeeYoung | 2020-05-21 06:01:46
As world-renowned pharmaceutical bio companies such as Gilead Sciences Korea, Novartis, and Pfizer have announced the development of COVID-19 treatment and vaccines, domestic pharmaceutical companies are also revealing their intentions through various mechanisms and strategies.
In particular, it has attracted attention by offering various possibilities from cytokine anti-inflammatory drug candidates through stem cells to drug repositioning and therapeutics that directly target the RNA genome of COVID-19 using RNA interference technology.
On the 20th, Bio Korea set up a special session on the development trend of COVID-19 treatment, and provided a place to promote the development of new drugs by sharing precautions for clinical and non-clinical development for treatment development and sharing cases of treatment development by domestic companies.
Six sessions of industry-academia, including academic research institutes such as the Institut Pasteur Korea and the Korea Research institute of Chemical Technology (KRICT), and Cellivery, which received attention as an in vivo transport technology for pharmacological substances, participated in this online session.
.Soon-wook Song, vice president of SCM Life Sciences, which first published a case study on the development of a stem cell therapy for treatment of COVID-19 (SCM-AGH), explored the possibility that stem cells could be used for COVID-19 treatment through anti-inflammatory functions
.▲Stop cytokines, Stem cell anti-inflammatory mechanism "There is a worldwide search for ways to use stem cells for the treatment of COVID-19," said vice president Song
."Currently, clinical trials registered with stem cells called Mesenchymal Stem Cells (MSCs) have been registered worldwide." The number of cases reached 17," he said
.
.It has been found in animal experimental models that stem cell administration reduces inflammatory cytokines that cause cytokine storms and activates immune T cells that are effective in suppressing viruses
.COVID-19 confirmed patients are exposed to the immune system's cytokine when the virus penetrates the human body, and the 'cytokine storm' phenomenon that attacks normal cells is expressed
."We have our own original technology for separating stem cells with high purity, and we are producing high-efficiency and low-cost stem cell therapies based on this technology," said Song
."We have already demonstrated its effectiveness in animal models of mild and severe acute pancreatitis with these high-efficiency stem cell therapies," he explained
.He said, "In particular, stem cells reduce the inflammatory cytokines TNF-alpha, INF-gamma, IL-1beta, and IL-6, which cause cytokine storms, and increase the anti-inflammatory cytokines IL-4 and IL-10
.And activated T cells
." Also he said, "The expected therapeutic effects of SCM-AGH stem cell therapy are also cytokine storm control," In addition, "regulatory T cells are activated to increase IL-10 secretion, so immune cells such as abnormally activated T- and B-cells can also be regulated." It is expected to induce efficacy
.Stem cell therapy is expected to induce the long-term treatment efficacy by rapidly normalizing the patient's immune system," he added
.Cellivery, which has recently emerged as a technology for in vivo transport of pharmacological substances, is also developing therapeutic agents with a focus on anti-inflammatory properties
.▲Regulate the signal of nuclear location in cells, Possible to suppress hyperinflammatory reaction Dae-Woong Cho, CEO of Cellivery, who announced 'Development of iCP-NI treatment for acute inflammation suppression', said, "Intracellular transmission of a nuclear localization signal can suppress inflammation by regulating the expression of cytokines / chemokines." "COVID-19 induces a cytokine storm, leading to death, and excessive inflammatory cytokines and chemokines can lead to destruction of bronchial and alveolar tissue, leading to permanent lung damage, such as lung fibrosis," he said
.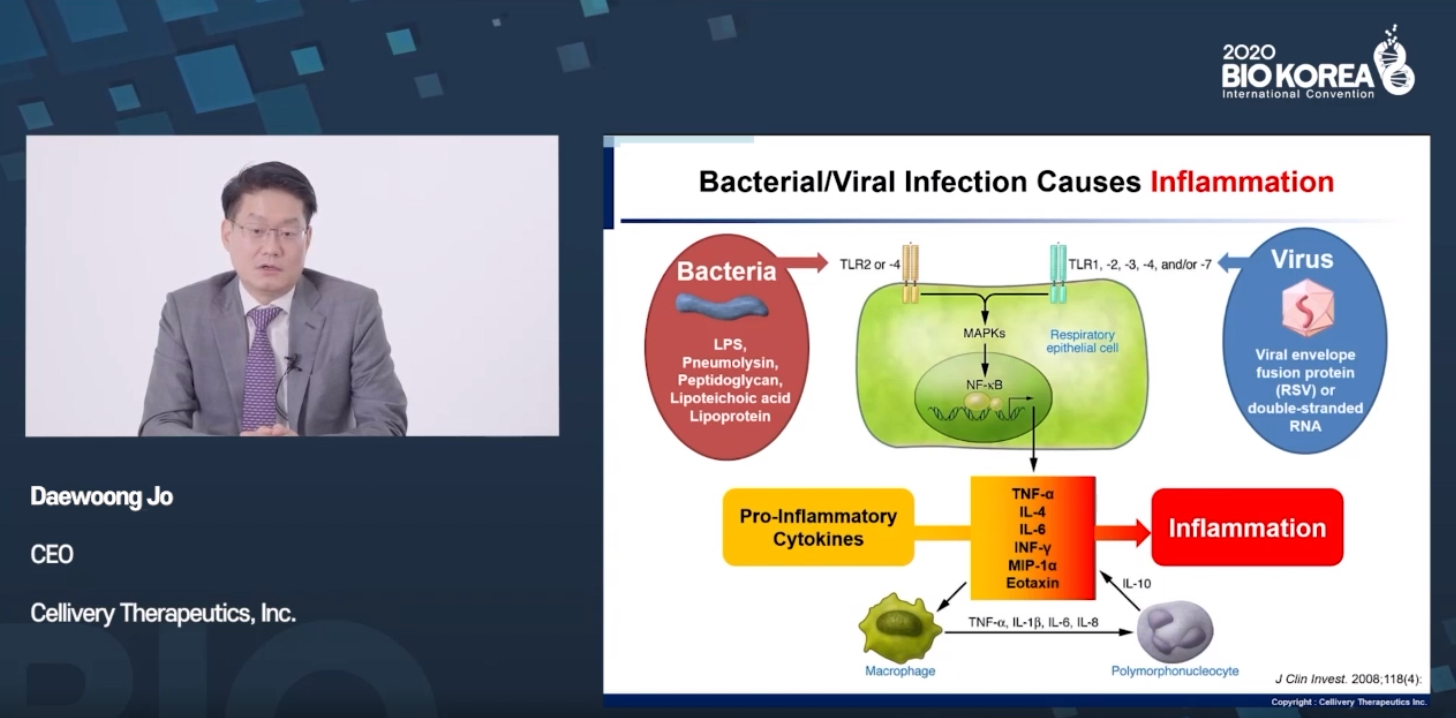
."The nuclear location signal transmitted into the cell prevents stress-responsive transcription factors (SRTFs) from moving from the cytoplasm to the nucleus
.It is a mechanism to limit and suppress the inflammatory response." As a result of animal experiments, iCP-NI showed the effect of regulating the expression of chemokines (MCP-1: -89%) and a cytokine (TNF-α: -79%, IL-6: -91%, IL-12: -110% & IL) in bronchial-alveolar fluid in an inhaled pneumonia model similar to RNA virus infection
.-10: + 574%)
.In addition, Bleomycin-induced pulmonary fibrosis in animal models reduced pulmonary fibrosis by 50% and iCP-NI protects leukocytes in staphylococcal enterotoxin B and acute pneumonia animal models (CD3+ T cell: 100%, CD4+/CD3+ T cell: 96%, B220+ B cell: 85%, CD45+ macrophage: 100%), and splenocyte cell apoptosis was reduced by 97%
.These results indicate that iCP-NI is a potential as a new treatment for inflammation of various infectious diseases such as COVID-19, which is accompanied by cytokine storms and severe sepsis
.▲Mutable RNA virus, solved by RNA interference Dong-ki Lee, CEO of Olix, announced the current status of COVID-19 RNA interference treatment
.The challenge for COVID-19 is the high rate of RNA-based mutations
.This is because vaccine development is not easy due to the possibility of mutation as well as resistance to therapeutic agents
."We are developing treatments for various diseases through our own RNA interference technology
.Among domestic companies, only Olix is entering clinical trials with RNA interference technology." said CEO Lee
.He explained that RNA viruses have a high variability, so they can easily develop resistance to therapeutic agents, but RNA technology can be used to directly target the viral RNA genome
.RNA interference technology has advantages such as targeting a region that is not prone to mutation, or minimizing the possibility of mutation by allowing multiple asymmetric small-interference ribonucleic acids (siRNA) to target the genome at once
.In addition, the Institut Pasteur Korea focused on 'Drug Repositioning' that utilize existing treatments in COVID-19
."There are currently no vaccines or treatments available for COVID-19, and drug repositioning is the only alternative," said Dr
.Seung-Tak Kim, researcher at the Institut Pasteur Korea
."To discover, we first screened about 3,000 antiviral drugs against the SARS coronavirus, which was prevalent in 2002 to 2003." He said that 48 FDA-approved drugs were selected and the drugs were tested for SARS coronavirus-2 antiviral activity again
.As a result, a total of 24 drugs with significant antiviral efficacy against SARS coronavirus-2 were found.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









