- LOGIN
- MemberShip
- 2025-12-24 21:54:02
- New reimbursed price for Imfinzi, Venclaxta and Blincyto
- by Kim, Jung-Ju | translator Byun Kyung A | 2020-03-20 06:25:01

Two treatments have accepted risk sharing agreement (RSA) terms set down on the negotiation table by Korea’s National Health Insurance Service (NHIS).
Amgen’s leukemia treatment Blincyto (blinatumomab) would lower its price by four percent considering the financial impact for increased reimbursed use with expanded indication.
According to pharmaceutical industry, Ministry of Health and Welfare (MOHW) is planning to revise the ‘List of Reimbursed Drugs and Maximum Reimbursed Price’ with the said changes.
The revision would come in effect from Apr.
1.
◆Imfinzi: The immunotherapy has been approved to treat unresectable, locally advanced NSCLC patients with no progression after platinum-based concurrent chemoradiation therapy (CCRT).
The company has submitted an application to Health Insurance Review and Assessment Service (HIRA) for reimbursement listing on Dec.
21, 2018, immediately after receiving an approval from Ministry of Food and Drug Safety (MFDS) in the same month.
At a Cancer Deliberation Committee meeting convened in early November last year, HIRA has decided it would be feasible to provide reimbursement on the drug.
The committee’s decision was based on clinical study proven clinical efficacy improved than placebo, and also the committee judged its cost-effectiveness would accept ICER value better than the placebo.
The refund and expenditure cap type RSA, proposed by the company, was at an acceptable level.

The drug negotiated drug pricing with NHIS from then on to last month As a result, the reimbursed price of the drug would be at 3,350,930 won per 0.5 g and 804,223 won per 0.12 g.
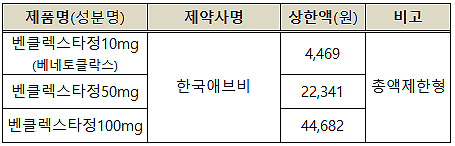
◆Venclaxta: Venetoclax is an orally administered anticancer treatment used to treat CLL as third or later-line treatment, and it is approved as a monotherapy for patients with relapsed or refractory chronic lymphocytic leukemia (CLL) who have been treated with chemo-immunotherapy and B cell receptor inhibitor, previously.
From last May last year, the pharmaceutical company has submitted a reimbursement application to HIRA, immediately after MFDS’ green light, and received a nod from Drug Reimbursement Evaluation Committee (DREC) in last December.
DREC official said the treatment has demonstrated clinical efficacy and is eligible for pharmacoeconomic evaluation (PE)-exemption track.
A drug can qualify for the PE-exemption track after qualifying all three conditions.
Then after, the company has successfully reached an agreement with NHIS last month on pricing negotiation under the expenditure cap type RSA.
The reimbursed price would be at 4,469 won per 10 mg, 22,341 won per 50 mg, and 44,682 won per 100 mg.
◆Blincyto: The anticancer treatment has been approved to treat adult and pediatric patients with relapsed or refractory Philadelphia chromosome-positive or negative immature B cell acute lymphoblastic leukemia (ALL).
From October 2016, the treatment has been provided with reimbursement in Korea for patients at age over 18.
The company has applied for expanded reimbursement to HIRA in February last year, after expanding its indication to treat adult and pediatric patients with relapsed or refractory Philadelphia chromosome-positive acute ALL in January last year.
At the June meeting convened last year, Cancer Deliberation Committee said selective reimbursement would be granted for remission consolidation therapy to reduce non-reimbursed criteria.
The treatment already has essential coverage on its remission induction therapy indication.
Its expanded reimbursement was finalized by Drug Reimbursement Evaluation Committee (DREC) in last December.
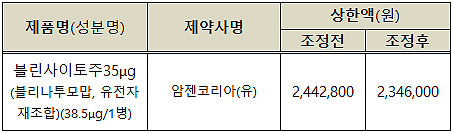
In this month, the company has finally reached an agreement on negotiated pricing with NHIS.
The key was to lower the price by four percent to lessen the financial impact.
The negotiated price was decided at 2,346,000 won, reduced from the original price of 2,442,800 won.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









