- LOGIN
- MemberShip
- 2025-12-24 21:54:06
- Stivarga renews RSA and Azilect lowers pricing by 30%
- by Kim, Jung-Ju | translator Byun Kyung A | 2020-02-21 06:36:09
By renewing an expiring risk sharing agreement (RSA) with National Health Insurance Service (NHIS), Bayer Korea has decided to lower the maximum reimbursed price for metastatic colorectal cancer and gastrointestinal stromal tumor (GIST) treatment Stivarga 40 mg tablet (regorafenib) by 7 percent.
And the health regulator has authorized pricing reduction on idiopathic Parkinson’s disease treatment Azilect tablet (rasagiline msylate) by 30 percent.
Korea’s Ministry of Health and Welfare (MOHW) plans to reflect the changes on the List of Reimbursed Drugs and Maximum Reimbursed Price.
Although most of the changes would come in effect from Mar.
1, some changes would be enforced from different dates due to individual issues.
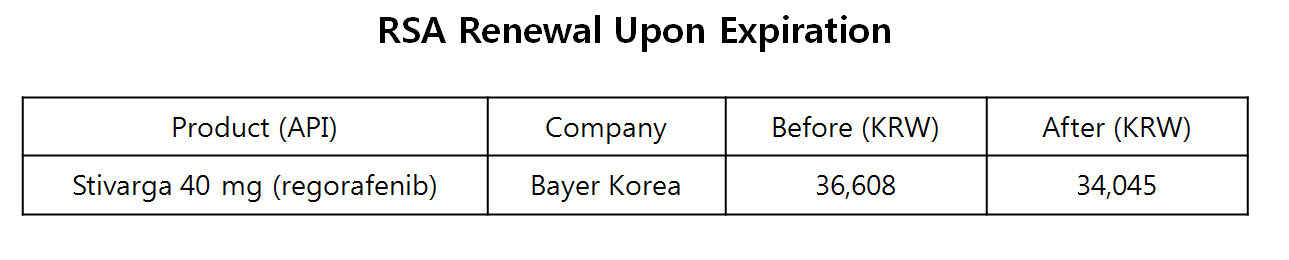
Stivarga 40 mg tablet took the refund type RSA track from June 1, 2016, and received reimbursement in Korea.
The original RSA, typically lasting for four years, was expected to expire on coming May 31.
The current reimbursed price is at 36,608 won, and the company has agreed to lower it by 7 percent to 34,045 won after renewing the negotiation.
The lowered pricing would be applied from June 1, when the renewed agreement comes in effect.
Accordingly, Korea now has three RSA-renewed drugs including Stivarga.
Merck’s metastatic colorectal cancer treatment Erbitux (cetuximab) and Astellas Pharma’s metastatic prostate cancer treatment Xtandi (enzalutamide) have also renewed their agreements.
The government manages post-marketing pricing with PVA for drugs exceeding initially estimated volume and claimed amount.
Under the PVA system, their prices are renegotiated and brought down.
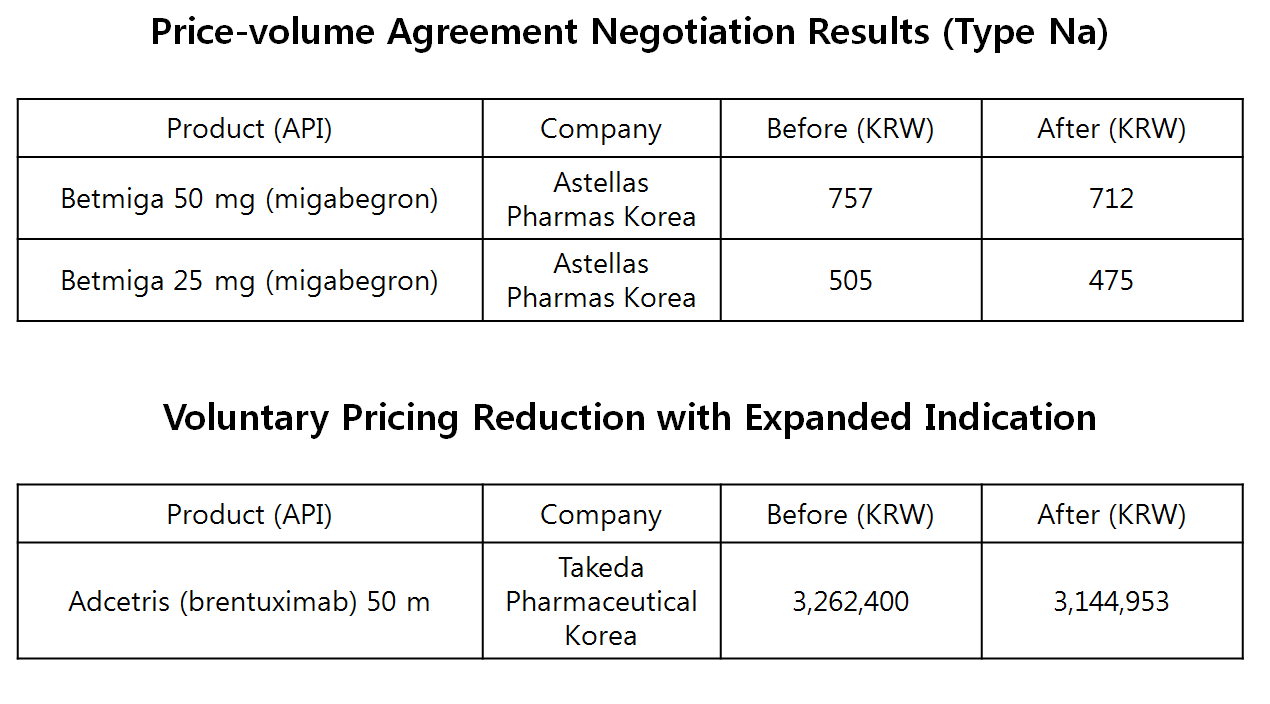
The tablet took the Type Na (나) PVA for the negotiation.
Type Ga (가) PVA applies to a new drug listed with pricing negotiation, but had claimed volume over 30 percent higher than the estimated volume.
For the agreement, the item has to have same company name, administration method, substance, and formulation as when it was first listed.
The Type Na PVA, applied to Betmiga, is for new drug either price adjusted by Type Ga or four years passed since listing date, and accumulated claimed volume surpassed the estimation by 30 percent.
It also applies when a drug’s accumulated claimed volume in a year is surged by 60 percent from the previous year, or is increased by 10 percent but the increased amount is over 5 billion won.
The negotiated pricing each lowered the original prices by 5.9 percent.
The maximum prices of 50 mg tablet and 20 mg tablet was dropped from 757 won to 712 won, and from 505 won to 475 won, respectively.
.The new pricing would take in effect from Mar
.1
.Meanwhile, another drug’s pricing would be lowered early due to expanded indication
.Based on analysis of estimated additional claimed volume and the increase rate, Takeda Pharmaceuticals Korea has agreed to reduce the pricing of Adcetris (brentuximab)
.Coming in effect from March, the current price of 3,262,400 won would be reduced by 3.6 percent to 3,144,953 won
.◆ Government-authorized changes: Due to generic’s reimbursement listing, total ten originals or drugs with same administration method, ingredients and formulation as an original would have their maximum price brought down by the government from Mar
.1
.When a first generic is listed for reimbursement, the government applies so-called ‘half-price,’ or 53.55 percent of the original’s price, on the generic
.But it applies weighted pricing of 70 percent of the original’s for the first year from the point of listing
.And the weighted pricing is sustained, even after a year, when there are less than three companies supplying items in the same class
.The pricing is kept until the number of companies reaches over four
.The prices of Lundbeck Korea’s Azilect 1 mg tablet and 0.5 mg tablet would fall by 30 percent from 3,501 won and 2,348 won to 2,451 won and 1,643 won, respectively
.And the price of Lily Korea’s osteoporosis treatment Forsteo (teriparatide) has also dropped by 30 percent from 326,358 won to 228,451 won
.Dong-A ST’s osteoporosis treatment Teribone SC injection 56.5 μg (teriparatide acetate) would be priced 22.2 percent lower from 73,287 to 57,001 won
.Except for Forsteo, other drugs’ weighted pricing would be eliminated from next Feb
.1
.The ‘half-price’ would be applied from then on
.◆ Raised maximum price and voluntarily-reduced drug pricing: Prices of seven items, designated as radiopharmaceuticals, would be significantly raised from Mar
.1 to secure consistent supply in Korean market
.The government either accepts raised maximum pricing of radiopharmaceuticals and National Essential Drugs, or compensates for the raised pricing by negotiating with NHIS
.The price of Korea Atomic Energy Research Institute’s Kaeri MIBG (1311) injection (2-iodobenzyguanidine (1311)) would be raised slight or exponentially by 0.4 percent of 120.6 percent, depending on the dose
.Some other items are voluntarily lowering their prices to better compete in the market
.The following six items are the case; Dong Kook Pharmaceutical’s antidepressant Dulcerin capsule (duloxetine hydrocholoride) in two doses, Dong Wha Pharm’s severe hand eczema treatment alitno soft capsule (alitretinoin) in two doses, Pharmbio Korea’s chronic pancreatitis quick symptom reliever Foichol 100 mg tablet (camostat mesilate), Hanmi Pharmaceutical’s HIV-1 infection and chronic hepatitis B virus treatment Tefovir tablet (tenofovir disoproxil phosphate)
.Dulcerin 30 mg and 60 mg capsules’ would be lowered by 4 percent and 6.7 percent, respectively
.Hanmi Pharmaceutical’s Tefovir tablet price would be lowered by 17.5 percent from 2,910 won to 2,400 won
.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









