- LOGIN
- MemberShip
- 2025-12-25 01:06:56
- Finally a complete revision of PE guideline after 8 years?
- by Lee, Hye-Kyung | translator Byun Kyung A | 2020-01-28 06:12:55
After long eight years, the Korean health authority seems to finally accept the pharmaceutical industry’s demand to amend the pharmacoeconomic analysis guideline.
Health Insurance Review and Assessment Service (HIRA) recently unveiled a final report on the cosigned research regarding ‘Pharmacoeconomic (PE) Analysis Guideline Revision Plan (Principal investigator: Professor Lee Tae-jin of Seoul National University Health Science Department and Professor Bae Eun Young of Gyeongsang National University College of Pharmacy).’ Aiming for a ‘complete revision’ of the PE analysis, HIRA has started the research from last year.
The research report, disclosed on Jan.
22, found countries the Korean PE analysis guideline refers to, such as the U.K., Australia and Canada, have entirely amended their guidelines after 2011.
And it claimed the Korean PE analysis guideline should also reflect the developed PE analysis methodology and precedents that the other countries have based since 2011.
The research team conducted a survey on stakeholders like pharmaceutical industry organization, patient and civic group, and related scholars, from July to September last year.
The team convened consultative meeting with Korean Research-based Pharmaceutical Industry Association (KRPIA), Korea Pharmaceutical and Bio-pharma Manufacturers Association (KPBMA), Korea Biomedicine Industry Association (KoBIA), Korea National Council of Consumer Organization, National Council of the Green Consumers Network, Korea Consumer Agency, National Health Insurance Policyholder Forum, Korean Association of Health Technology Assessment (KAHTA) and other experts.

The team recommended the guideline to remove ‘financial impact’ article, but to add ‘statistical consideration (estimated long-term effect, treatment switching (cross over) and etc.)’ and ‘analysis guideline for laboratory test drug.’ ◆ Cost: As the article of cost in PE analysis depends on the perspective, the researchers recommended using healthcare perspective instead of the existing limited societal perspective, as well as only including direct medical expense and excluding non-medical expense (transportation cost, time cost, nursing guardian cost and etc.) for the basic analysis.
In the PE analysis material submitted to this date, expert’s opinion takes up the majority of data source with seven cases (14 percent) in diagnostic cost and five cases (ten percent) in treatment cost.
Compared to data-focused referential material, expert opinion or cost and resource use quoted from hospital investigation have limited credibility or consistency.
Therefore, referencing expert opinion should require official statement of an academic society, or stipulated minimum number of expert panel to improve credibility and consistency.
Although the existing guideline does not mention drug wastage, the researchers suggested stating the cost of wastage volume, because it generates noticeable amount of expense.
◆ Utility, the healthcare related quality of life: Rounding up the recommendations made by HIRA, pharmaceutical companies, and civic groups, the researchers said following articles in the guideline about ‘utility’ should be revised; prioritization of utility measuring techniques; Health-related quality of life (HRQL) indicating tool and reviewing tariff selection; detailed guideline on mapping; detailed guideline of direct measuring; and additional review on minimal clinically important difference of utility.
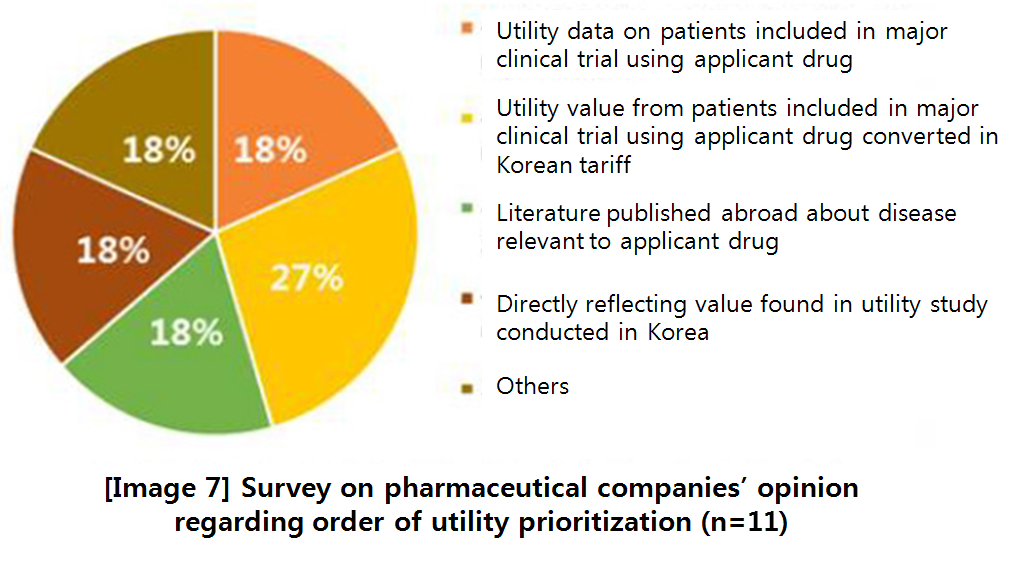
About reviewing and selecting HRQL tool and tariff, they argued the health authority should select a single set of tool and tariff to ensure consistency in policy-making process.
‘Tariff’ means the value that converts utility value in foreign country to fit the Korean landscape.
The primary recommendation is to select EQ-5D-3L, a tool used the most home and abroad so far, as a single tool and to select tariff developed in the most recent study ‘Lee et al.
(2009)’ with the largest sample as a standard tariff.
However, the team also proposed keeping the existing guideline as a secondary option, because a proper comparison study or qualitative evaluation on HRQL tool and tariff in Korea has not been conducted, yet, and related empirical data are insufficient.
◆ Discount rate: According to 50 cases of PE analysis material submitted to HIRA, 45 cases (90 percent) have been discounted and five cases (10 percent) have not been discounted.
The analysis period of the five cases was apparently under a year.
The researchers stated the discount rate for PE analysis should be reduced as well, because the social discount rate continues to descend due to changes in socio-economic conditions.
Hence, the report suggested the revised guideline should set 4.5 percent as an adequate level of discount rate, equivalent to the discount rate applied on preliminary feasibility assessment.
About applying the same discount rate on the cost and result, the report tried to persuade applying the unchanged rate, but applying either zero percent for no discount or three percent discount rate to analyze sensitivity.
Applying the discount rate of 4.5 percent for the entire period of basic analysis was suggested in case the period of PE analysis for the healthcare sector is longer than three decades.
But also as a sensitivity analysis, 3.5 percent could be applied after 30 years in case the applicant drug is essential for pediatric treatment.
◆ Perspective: Taking in account the state and realistic environment of reimbursement decision making, the researchers argued the analytic perspective should be amended.
Keeping in mind the interest of reimbursement listing reviewer is on the impact on the healthcare sector, the research team first recommended shifting the perspective from limited societal perspective to healthcare perspective and excluding items applicable for direct medical cost from the basic analysis.
So the cost should cover items centering the direct medical expense, but it should include nursing if the cost is reimbursed from long-term nursing insurance, whereas the effect should reflect benefit of health on the patient.
The secondary proposal suggested was maintaining the current guideline.
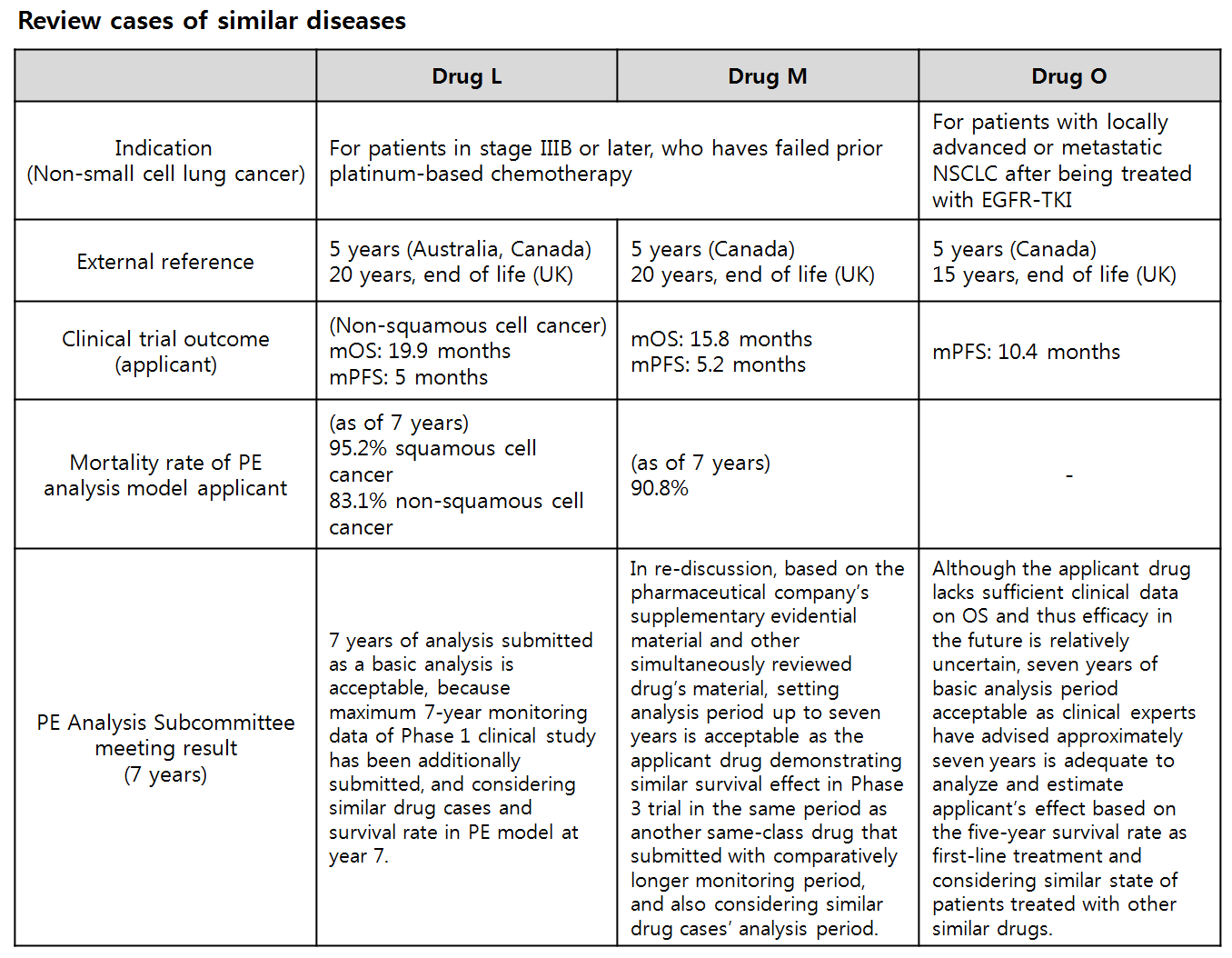
The research team studied deliberation cases of similar diseases and contemplated on patient age (cohort joining age), expected life expectancy of general population group, patient’s survival rate, analysis period of similar drugs, clinical trial outcome (median OS), monitoring period, clinical consulting, and uncertainty for calculating analysis period.
The team advised to maintain the principal approach of the existing guideline, but to consider validity of calculated analysis period.
Also the team elaborated the revision, when comparing with other countries’ guidelines, should take in account the age of applicable population, life expectancy, survival rate based on epidemiological data, median OS observed from clinical trial, difference in survival rate between cohorts, monitoring period, numerous uncertainties inserted in model, similar drug evaluation case or cases in referential countries and expert consulting.
◆ Analytic techniques: The current guideline recommends using cost-utility analysis when quality of life is a crucial aspect or the health outcome is demonstrated in various indicators.
But the research team claims the guideline should provide more specific standard or analytic techniques to distinguish equivalence of effect.
They first advised offering more detailed guideline on selecting subject for cost-minimization analysis, expense item and submission data to be considered when conducting the cost-minimization analysis.
Regarding subject for cost-minimization analysis, the researchers recommended clarifying the cases of the drug effect is non-inferior (or superior) and recognizing the non-inferiority of the applicant drug in safety profile.
As for the cost estimation, the guideline should set equi-effective doses and provide cost of comparing options’, as well as other costs estimated from monitoring, adverse reaction relief, and others.
The secondary recommendation is to exclude cost-minimization analysis from the analytic techniques.
The researchers explained it would theoretically eliminate the issues of effect equivalence and uncertainty.
◆ Subject of comparison: The existing guideline stipulates selecting alternative treatment option with the highest market share as a reference.
When there are multiple drugs with similar market share, multiple options can be selected for comparison, and other therapy and surgery can be used as reference if there is no other alternative option.
The referential option selection has been an issue raised by the pharmaceutical industry for a long while.
The Korean guideline clearly states to select a substitutable option with the highest market share, but many of other countries’ guideline does not specify “the highest market share.” As a primary recommendation, the researchers suggested keeping the basic principal of selecting the ‘most used’ drug for comparison, but to loosen the description of ‘highest market share’ to prioritize replaceability when selecting a referential treatment option.
The majority of the researchers were negative about providing a range of options like in Australia or limiting the option to reimbursed treatment, but they agreed on having government committee to discuss or negotiate with pharmaceutical companies based on submitted PE analysis material and market status.
However, the team put down a precondition that comparing with existing treatment option, which fails to treat health condition but has no other option, is ‘not automatically neglected from referential candidate list.’ ◆ Data source: The HIRA’s guideline barely mentions of indirect comparison, but a separate indirect comparison guideline is used to accommodate, and the government agency has already opened up about possibly accepting simple comparison of drugs as they reflected stakeholders’ demands, since the 2014 revision.
The researchers elaborated “It could be seen that it was actually more lenient than other countries’ guidelines,” and advised that the revised guideline should edit the detailed guideline and supplementary explanation in a wider sense.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









