- LOGIN
- MemberShip
- 2025-12-25 02:52:55
- What criteria does the Pharmaceutical Committee judge?
- by Lee, Hye-Kyung | translator Choi HeeYoung | 2020-01-14 08:49:17

The HIRA evaluates the adequacy of a drug through a review by the Pharmaceutical Benefits Evaluation Committee in accordance with Article 11 (2) of the Rules on National Health Insurance Benefits.
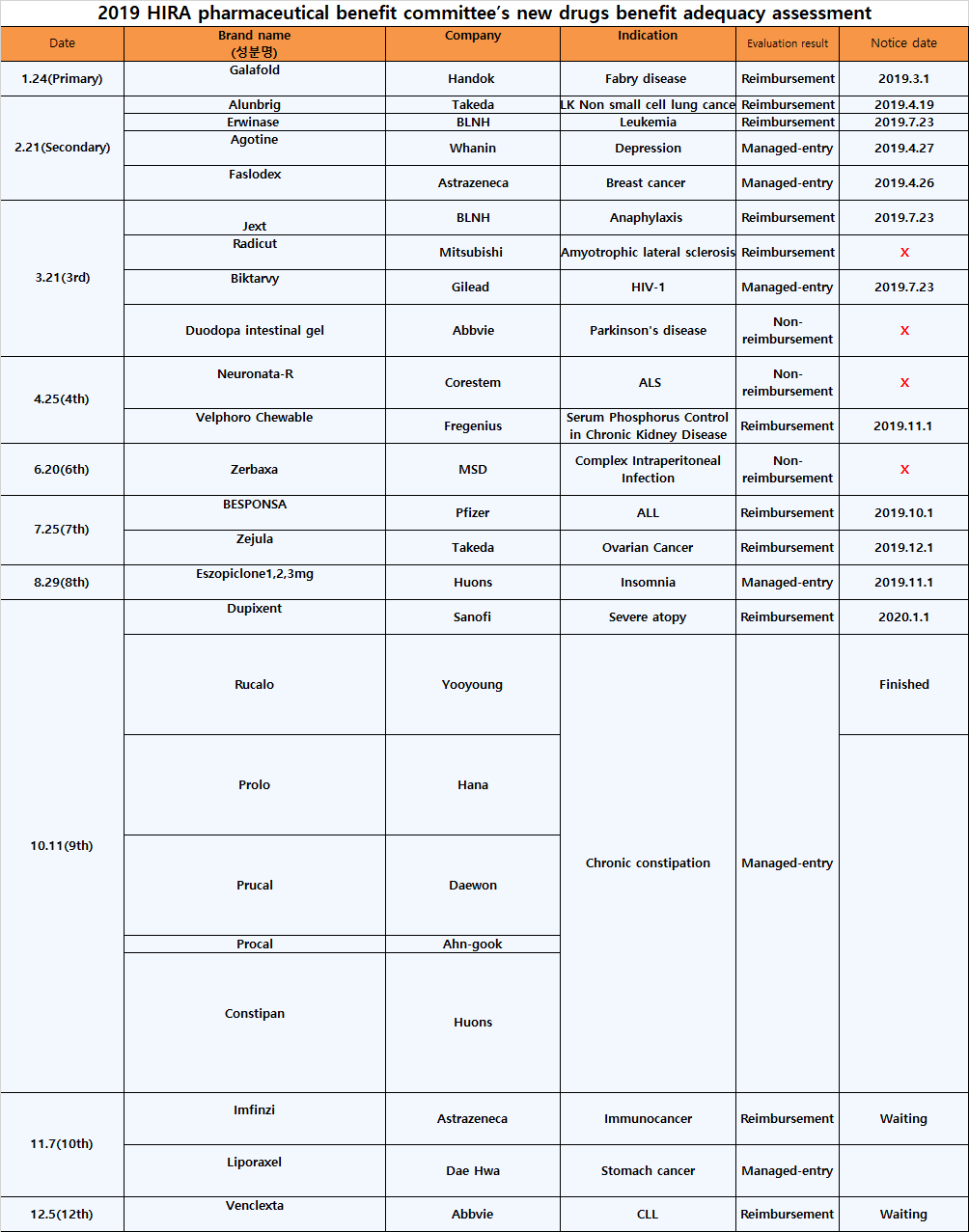
Dailypharm analyzed new drugs that passsed by the HIRA's Pharmaceutical Benefit Committee from January to December last year.
Of the 24 drugs, only 11 received reimbursed.
The remaining 10 drugs were non-reimbursed, and 3 drugs were managed-entry.
.Non-reimbursed drugs include 'Duodopa intestinal gel' (levodopa)' by Korea's AbbVie, 'Neuronata-R(autologous bone marrow-derived mesenchymal stem cells)' by Corestem, and 'Zerbaxa' (Ceftolozane/Tazobactam) by MSD Korea
.Radicut(Edaravone), the treatment of amyotrophic lateral sclerosisby Mitsubishi Danabe, chose to withdraw its reimbursement during the drug price negotiations with the HIRA
.
.However, the clinical data submitted by the pharmaceutical did not qualify as 'medical drugs deemed necessary for medical treatment' because clinically meaningful improvements such as prolonged period of survival have not been proved
.As a result, it was concluded that ICER was impossible due to insufficient evidence, and drug prices suggested by the company were difficult to accept due to uncertainty
.On the other hand, the related academics said that patients who are not controlled by existing Parkinson's disease drugs because they do not have adequate treatment in Korea are necessary for patients suffering from mental and physical pain and severe diseases due to exposure to medical blind spots
.This drug is listed in the US, Germany, France, Italy, the UK, and Japan excluding Switzerland among A7 countries
.In patients with amyotrophic lateral sclerosis, clinical necessity was acknowledged by slowing functional decline than without Neuronata-R, The cost was higher than that of the alternative, and the corresponding cost effectiveness was unclear, and A non-reimbursement decision was made
.The domestic neurology textbook mentions the approval of rare drugs in the applied products, and the stem cell treatment is introduced as a treatment under study in the ALS in clinical guidelines
.However, Yakyeong said, “Riluzole is being given to Amyotrophic lateral sclerosis, and considering the possibility of substitution, it is hard to say that it is an essential drug for medical treatment”
.The related academics also suggested that it would be advisable to review the Neuronata-R strains after the results of phase II and III studies have been reported
.
.The deliberation was conducted on November 21 2018, through the Pharmaceutical Benefits Subcommittee, and the deliberation was conducted by the committee on last June 20
.As a result of the deliberation, the clinical necessity is hardly considered inferior to the clinical treatment rate, but it was concluded by non-reimbursement due to the higher cost than the alternative drug
.The relevant academics suggested that the introduction of an inferior therapeutic agent compared to Carbapenem has a clinical significance in the case of limited antibiotics against MDR-infected Gram-negative bacterial infections
.Among A7 countries, Zerbaxa is registered in the US, Italy, the UK, and Japan
.
.Radicut was approved as a drug that could slow the progression of dysfunction caused by amyotrophic lateral sclerosis, but the clinical necessity was recognized, but the cost was high
.However, there are no drugs or treatments with the same therapeutic position, and it has passed the severity and social impact of the disease according to the risk sharing plan and the policy to strengthen health insurance coverage as a rare disease treatment drug
.The problem was that Mitsubishi Danabe Pharma had been discussing reimbursement in Canada at the same time as Korea
.However, Canada decided to refer to Korean drug prices, and withdrew Korea's reimbursement entry to enter Canada's reimbursement
.At that time, Radicut was listed in the US and Japan among A7 countries
.On the other hand, in order to be reimbursed again, submissions of new cost-effectiveness data should be evaluated and submitted to the Pharmaceutical Benefit Committee
.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









