- LOGIN
- MemberShip
- 2025-12-25 01:07:01
- Generic pricing should be at original’s 40%-45%
- by Lee, Hye-Kyung | translator Byun Kyung A | 2020-01-08 06:17:56
The national insurer’s research result claims the volume of generic would increase up to 73 percent to 82 percent if the use of generic is promoted, if the present lump-sum generic pricing reduction is brought down to 40 percent or 45 percent of the original price before patent expiration.
Currently, the lump-sum pricing reduction drops generic pricing to 53.55 percent of the initial pricing of the original.

Korea’s National Health Insurance Service (NHIS) recently published a final report of a year-long cosigned study of improving drug supply and purchase program on All Public Information In-One (ALIO) conducted with Sungkyunkwan University’s academic-industry cooperation research team (Principal researcher: Professor Lee Sang-Won of Pharmaceutical Technology & Business Management Department at School of Pharmacy).
Also known as ‘Pharmaceutical Product Life-cycle Research Report,’ the study was directly ordered by President Kim Yong-ik of NHIS, who spent 250 million won on the massive research project.
According to the report on Jan.
6, the research team has laid down regulatory policy recommendations in categories of pharmaceutical supply structure, generic supply structure, pharmaceutical distribution structure, and Korean-made new drug supply structure.
◆ Pharmaceutical supply structure: The university research team suggested reforming pharmaceutical supply structure to secure financial health of National Health Insurance (NHI), to supply good quality drugs and to expand Korean generic items’ market share.
To achieve the goal, the report recommended seven objectives in three categories; improving generic supply structure (generic quality standard strengthening policy, off-patent drug pricing reduction policy, generic use promoting incentive policy); distribution structure (distribution company competitiveness enhancing policy, fair trade order reinforcing policy); and new drug supply structure (corporate R&D investment expanding policy, technology commercialization supporting policies).
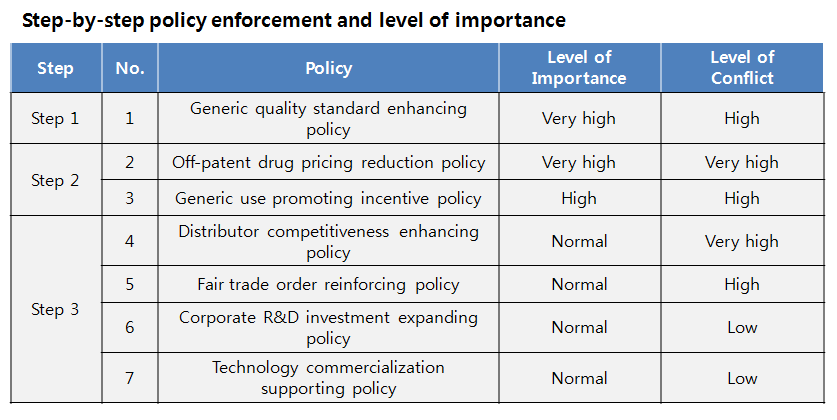
Starting from generic quality standard enhancing policy, Step 2 off-patent drug pricing reduction policy and generic use incentive policy and Step 3 distribution structure and new drug supply structure shifting policies should follow, the researchers claimed.
The Step 1 objective, generic quality standard enhancing policy, was prioritized as it affects the three policy goals overall, which are ‘NHI financial health,’ ‘outstanding drug supply’ and ‘self-sustained medical technology.’ Policies targeting distribution and new drug supply have been assigned under Step 3 objective, because their importance level was apparently lower than that of generic policies.
The researchers commented, “The importance of generic quality standard enhancing policy and generic drug pricing reduction policy are very high and they require intricate plan of action.
Also they need to consider each interest group’s stance.” ◆ Generic supply structure: The research team stated the present volume of generic use in Korea (61.8 percent as of 2017) should meet the average level of OECD member countries (over 70 percent) to leverage generic supply structure and industry.
To realize the goal, bringing down the generic pricing is essential.
The report calculated the ratio of generic use in overall drug volume could be raised up to 73 percent to 82 percent, assuming generic use would soar when the price is dropped to 40 percent to 45 percent of the original’s initial price.
Previously, the researchers mentioned of three policies—generic quality standard enhancing policy, off-patent drug pricing reduction policy and generic use incentive policy—to achieve the aim to reform pharmaceutical supply structure.
The policy objectives of reforming generic supply structure is to lower pricing to the level of competitive market and increasing volume on par with advanced countries, while enhancing the quality of generic items.
More detailed objectives of enhancing generic quality includes tightening management standard change after approving and conducting stringent Good Manufacturing Practice (GMP) due diligence.
As for the mechanism of drug pricing reduction, the report recommended reforming actual transaction price (ATP)-based drug pricing reduction and lowering drug price on the level of competitive market.
For the ATP-based pricing reduction, the study argued the existing system of calculating reduction rate by each item should be changed to calculate by same class medicine.
The report elaborated the change would reduce not only a specific item’s pricing, but affect all items in a same class, which would enable supply of low-priced drugs.
Then sales of a company with capacity to supply mass amount of low-priced products would surge and eventually push up the efficiency of NHI expenditure management, the researchers added.
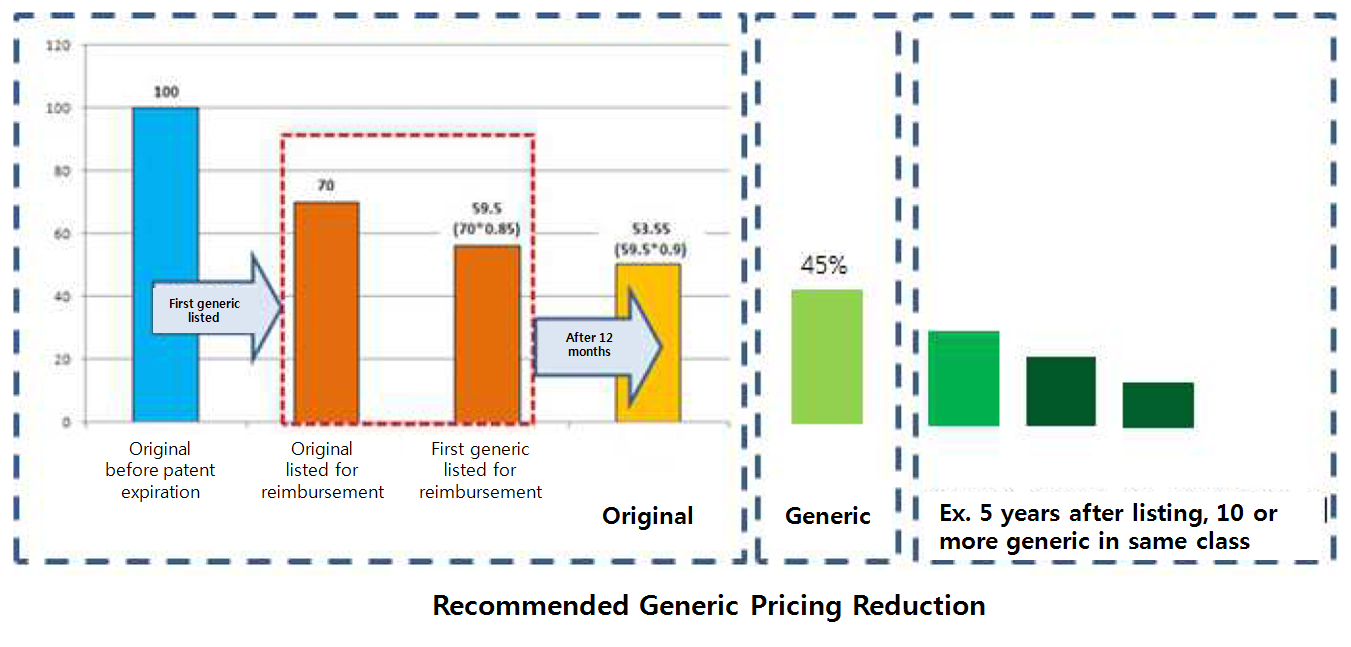
Basically, the generic pricing would not reach the level of off-patent original pricing.
The report added another recommendation to constantly monitor pricing of off-patent drug market and regulate pricing reduction on generic listed for a long time or with pricing unchanged despite multiple listed generics.
After enhancing generic’s quality and lowering pricing, incentive policy is recommended to promote further active use of generic.
Increasing demand for low-priced drug in the market would ultimately expand volume of generic use and promote price competition.
To increase the demand for low-priced drug, the report proposed the following; providing incentive for using more generic to doctor and pharmacist; reforming payment system to adopt capitation; prescription budget cap; or diagnosis related group payment; and lowering patient copayment or providing incentive point.
On the other hand, the report also proposed increasing generic use by the purchasing power of the insurer, NHIS.
Apparently, the insurer could select and announce a generic item capable of supplying low-priced product among multiple generic items in equivalent class, or set and mandate alternative prescription goal for pharmacist on preferred generic item.
◆ Pharmaceutical distribution structure: The researchers claimed the currently convoluted distribution models should be better sorted out.
Depending on a distributor’s business management and scale, the type of distributors should be divided into ‘Total Distributor (owning a nationwide sale network and dealing logistics and commercial distributor),’ and ‘General Distributor (commercial sales),’ and ‘Contract Sales Organization (CSO).’
And the researchers argued idealistic image of Korean drug distributor should be set forth by establishing distributor evaluation index and designating an Innovative Distribution Company based on ‘Special Act on Fostering and Support Pharmaceutical Industry,’
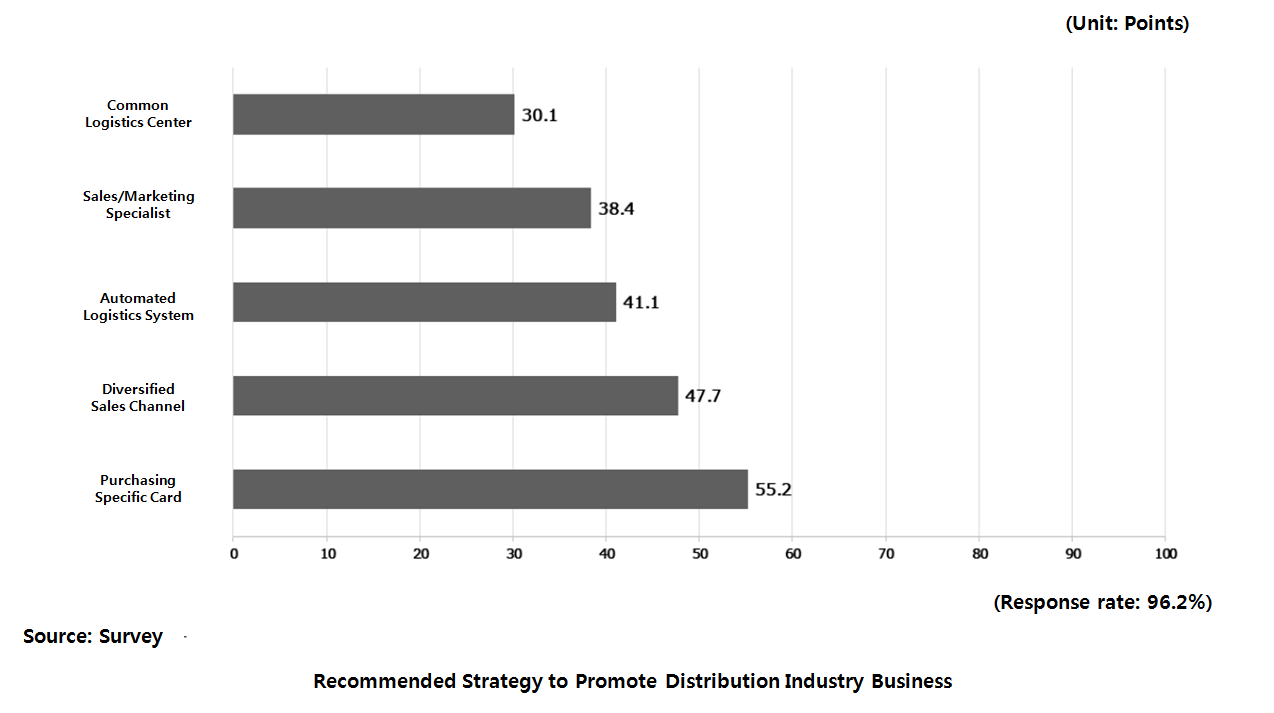
Besides, the report presented tasks to reform structure, regulation and environment of the pharmaceutical supply by adopting ‘Pharmaceutical Purchasing Card’ to set straight wholesale profit margin inflated with unreasonable distribution cost; forming ‘Supply Chain Council’ with pharmaceutical company and distributor’s representative to talk about minimum profit margin; enhancing logistics and minimizing return through improved minimum packaging unit and labeling; and requiring aggregation; fostering distribution and marketing specialists to advance distribution industry.
◆ Korean-made new drug supply structure: The research team stated the supply structure of new drug developed in Korea should be strengthened in both quality and quantity by inducing innovativeness of new drug, and by setting a reasonable supply volume goal based on global level productivity of new drug.

To expand supply of Korean-made new drug and improve its innovativeness, the researchers proposed reinforcing Innovative Pharmaceutical Company support system, changing new drug R&D policy, supporting open innovation and fostering development and commercialization capability.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









