- LOGIN
- MemberShip
- 2025-12-25 01:06:59
- Announcement of drug price reform is delayed
- by Kim, Jung-Ju | translator Choi HeeYoung | 2020-01-04 20:47:33
The reform of the drug price system, which the government plans to announce last month, is delayed somewhat.
The pharmaceutical industry is all on edge because of the reorganization of generic drug prices, improved risk-sharing contract (RSA), economic exemption system, and insurance policy supplementary agreement (sub-contract).
◆Generic price restructuring= Among the many cases where drug price restructuring is expected, the focus of the industry is on the final revision of the 'Determination and Adjustment Criteria for Drug Price Revision', which contains the generic price restructuring.
The government planned to announce the final announcement no later than last month, but it was slightly delayed, with some revisions to the earlier this month.
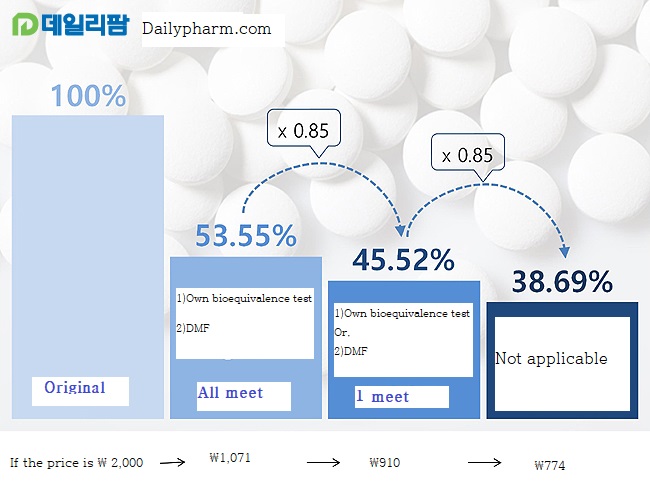
It is largely divided into generic cascade drug price reform and cut line system.
The requirement for a cascading drug price reform is to conduct its own bioequivalence test and to meet the registered use of the Drug Master File (DMF).
If both criteria are met, the price is estimated at 53.55% of the cost of the original drug (before generic listing).
However, if one or not met, then the price is multiplied by 0.85 based on 53.55%, depending on the level of fulfillment of the criteria.
They are cut by 15%.
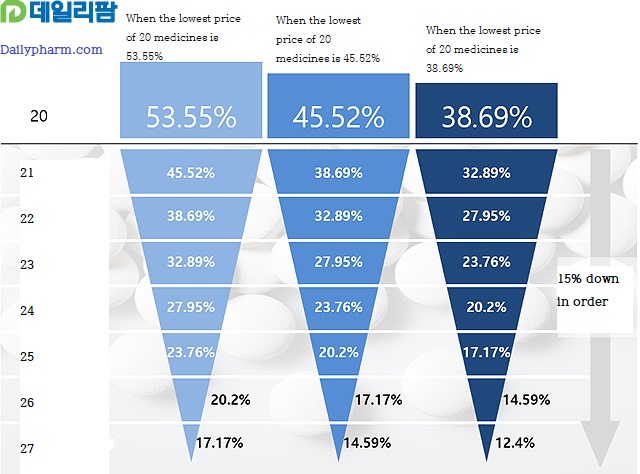
Starting from the 21st order of health insurance, the drug price is estimated at 85% of the lowest price regardless of whether the criteria are met.
For example, the 21st generic is estimated at 85% of the lowest price in 20 products, while the 22nd generic is 85% of the 21st generic price.
The government has announced that the drug price reform will be finalized by the middle of this month and planned to be implemented in July.
◆Completion of RSA, reimbursement decision, the economic evaluation exemption system and additional contract creation subjects=In the current drug price system, issues that require expansion or reorganization of the reimbursement gateway stage are also overhauled.
The government has continually improved the drug price system without changing the fundamentals of the system since the implementation of the positive list system.
However, as the groundbreaking reinforcement of security continued, the awareness of the need to reorganize and upgrade the positive list system continued.
The improved drug price system can be divided into: ▲expanding the scope of RSA coverage ▲complementary details of reimbursement determination ▲complement of economic evaluation exemption system ▲expansion of generics for writing subsidies when reimbursement listed.
First of all, in the case of RSA improvement, one of the new drug listing tracks, it is important to expand the gateway to be able to apply the RSA target criteria that could be applied only to the current selection drugs.
In the meantime, drugs that have the same therapeutic position but have not been covered by RSA just because of late-release drugs will have a wider benefit opportunity.
In addition, high-priced drugs that are subject to phase III conditional approval are considered to have greater uncertainty, and thus, improvements are made in the direction of raising benefits by reimbursed them within the framework of the RSA.
In the case of reimbursement determination, some socially disturbing reimbursed priorities are supplemented.
The government is currently making reimbursement in consideration of medically feasible or medically significant materiality and treatment effects, as well as patient costs and social benefits.
In the future, it will be added to determine detailed principles or priorities in consideration of the financial status of health insurance.
In other words, it reflects the decision of reimbursement to the financial level that health insurance can cover, along with the characteristics of pharmaceuticals and the necessity of the times.
The economic evaluation exemption system will also be supplemented.
The government plans to improve the system by adding antibiotics, tuberculosis treatment, and emergency detoxification drugs, which are difficult to evaluate, to reflect the voice of expanding the scope of drugs that are difficult to evaluate.
Lastly, it is a revised plan that includes a generic agreement that is included when insurance is listed.
The contracting parties are the Korea Health Insurance Corporation and pharmaceutical companies.
The main contents of the contract are to fulfill supply obligations and accept price cuts when disposing of rebates.
The government aimed to implement the midterm this year after outlined this month's reorganization plan based on improving the positive list system and promoting administrative notice.
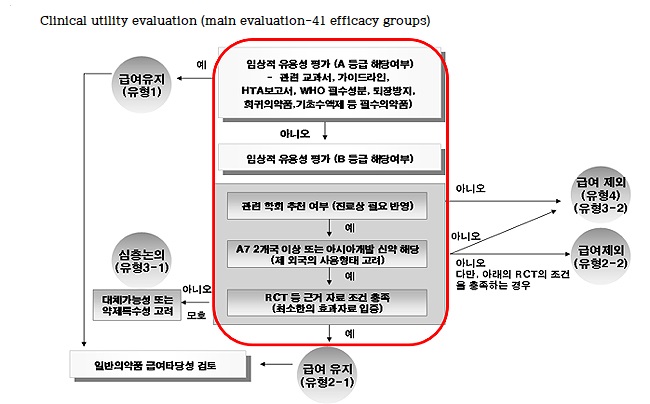
The evaluation is based on the revised List of Contracts, which was carried out 12 years ago, but the standard is expected to be more detailed.
Reevaluation targets are selected from anti-cancer drugs, rare disease drugs, and drugs of uncertain clinical usefulness.
Of these, ▲drugs that need to confirm clinical usefulness through effects re-evaluation ▲drugs that need to be managed due to increase in population structure and usage ▲other drugs that require evaluation taking into account social impacts and other effects on health care at ex post evaluation Sub-committees become standards.
The government plans to announce the final reassessment soon and finalize the system within the year.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









