- LOGIN
- MemberShip
- 2025-12-25 01:06:57
- 'Brakes' on reimbursed drug’s listing process
- by Lee, Hye-Kyung | translator Choi HeeYoung | 2019-12-27 06:27:27

Last year, The NHIS chairman Yong-ik Kim, who designed Moon Jae-in Care, was very active, and this year, there have been a number of institutional improvements that have been carried out by staff in charge of implementing the policy.
In the drug sector alone, there were many big and small things happening, including the expansion of screening benefits and RSA drugs, and the creation of contracts that included supply obligations prior to drug price negotiations and patient protection and confidentiality.
As the HIRA manages the health insurance finance, it has to focus on the income expansion to support the policy of strengthening security and the task of spending expenditure efficiency.
If the focus has been on finding new drug-based follow-up management plans, next year, it will continue to seek ways to improve the 'price-volume agreement' that has been spinning the wheels with research services several years ago.
The HIRA reviewed the revised economic evaluation guidelines and reference methods for foreign drug prices until the second half of this year to improve the drug listed system.
In August, the RSA target was expanded from cancer and rare diseases to severe and intractable diseases, and the severe atopic dermatitis, Dupixent, will be paid from next January.
◆Enhancing Access to Medicines by Moon Jae-In Care= This year, the first case of drug screening benefits was released.
In order to strengthen health insurance coverage, six types of anti-cancer drugs, which had no reimbursement standard or remained as 'baseline non reimbursement' at 100% of the copayment rate, were selected as a screening benefit.
The protagonists of the first screening benefits include breast cancer treatments ‘Perjeta (Pertuzumab)’, ‘Halaven inj (Eribulin)’, Prostate Cancer Therapeutics, ‘Xtandi soft cap (Enzalutamide)’, and ‘Zytiga (Abiraterone)’.
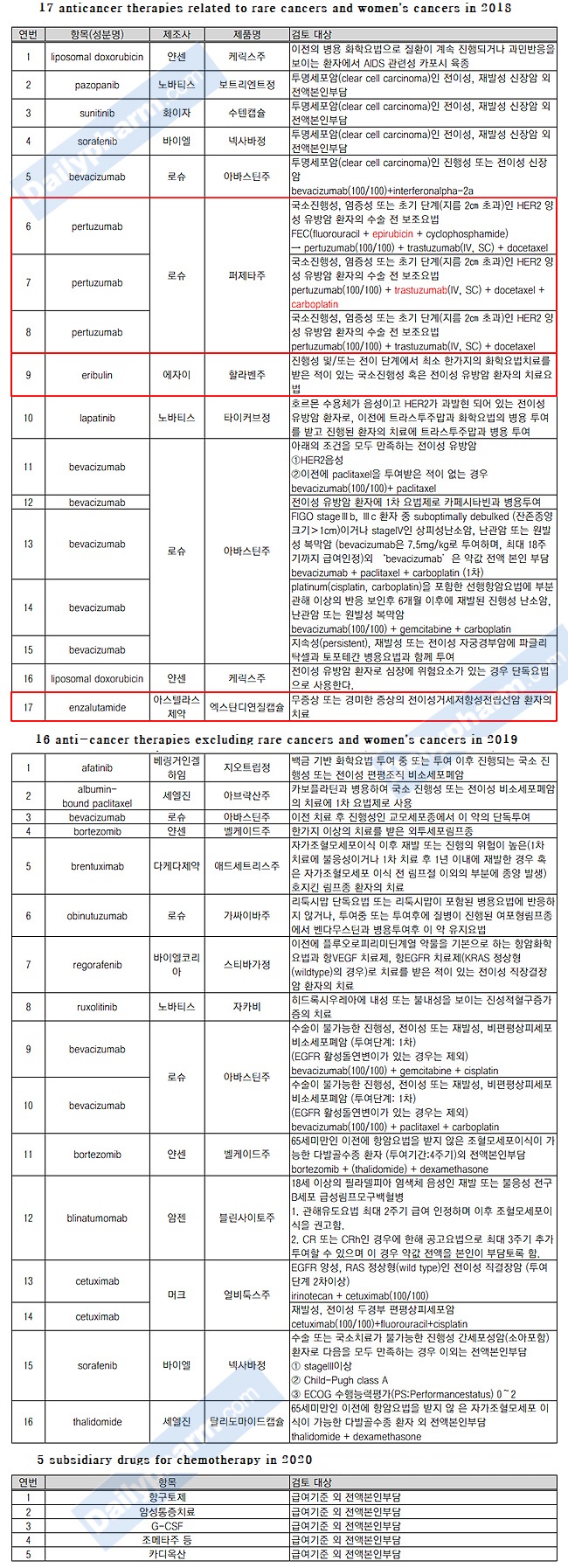
Dupixent, which was not an anticancer drug or a rare disease treatment drug, had difficulty in applying RSA expansion, but the health insurance policy review committee decided to complete the reimbursement registration process on the 23rd.
The MOHW applies a special calculation to reduce the incurable cost of medical expenses (20~60% → 10%) for rare and incurable diseases, and expanded the target diseases.
In January of this year, The MOHW identified 100 rare diseases and included them in the exception, and added 91 new rare diseases in the second half of this year.
In the case of medicines, health insurance coverage is being expanded to focus on the treatment of serious diseases such as anticancer drugs and rare diseases.
Compared to 2016, anti-cancer drug spending rose 41% from ₩1.47trillion to ₩1.46trillion in 2018, and rare disease treatment spending increased 81% from ₩235.2 billion to ₩4265 billion.
During the same period, the drug cost growth rate exceeded 19%.
This year, a new high-priced drug called Spinraza, which costs ₩560 million for the first year and ₩280 million from the next year, will cost ₩92.35 million per vial per patient.
It is also a significant year for reimbursement listing under both total contracts system and RSA.
◆Reorganization of departments related to drug price in the HIRA=The organizational reform of the department in charge of drug prices in the HIRA, which was in charge of the implementation of Moon’s care, was an issue.

Dr.
Jong-Heon Park, former researcher, was appointed as the head of the reimbursement strategy division.
Since the establishment of the Drug Price System Division, it has also lowered the threshold of 'secret drug price negotiations' by opening a foreign drug price inquiry guidelines bulletin board and opening the drug price negotiation drug.
In July, the glass ceiling of the 2nd level pharmacy occupation promotion of the HIRA broken.
In order to guarantee the opportunity for promotion of pharmacy jobs, which was stopped at Level 3, the HIRA has been reorganizing its personnel and organizational regulations since 2017.
As a result, Nam-sun Shin, the head of drug price negotiations, a second-level manager was produced from the pharmaceutical industry.
◆The HIRA, fix the drug price system= Last year, the Economic Evaluation System Improvement TFT was operated to revise the economic evaluation guidelines for medicines.
TFT focused on the comparative drug, ICER, utility, and discount rate that the pharmaceutical industry has pointed out.
Based on the TFT report, the HIRA is publishing commissioned research announcements to develop guidelines and conducting research.
The economic evaluation system was created in 2007 following the introduction of a drug screening system.
Over the past decade, the Economic Evaluation Subcommittee of the Committee on Drug Benefit Evaluation has reviewed data of approximately 190 ingredients (80 times) based on economic evaluation guidelines.
However, since the guidelines used to evaluate the economic feasibility have been applied since the beginning of June 2006 and only one revision was made in December 2011, the pharmaceuticals complain that the reality is not reflected.

At the time of the face-to-face screening process, 90-100% of the weighted average price of alternative drugs was accepted and drug price negotiations were skipped.
Among the drugs envisaged by the Health Insurance Policy Review Committee, there was a brake on reimbursement such as antidepressant Agotine tablet of Whanin, Faslodex of breast cancer treatment of AstraZeneca Korea, and Alunbrig tablet of non-small cell lung cancer treatment of Takeda.
This is because members of the Health Insurance Policy and Deliberation Committee have put in place conditions for the drug price agreement.
The conditional resolution was foreseen to some extent from the time the Ministry of Health and Welfare switched all the drugs proposed to the Health Policy Review Committee to face to face examination.
The MOHW raised the CJ Healthcare's K-cap tablet for gastroesophageal reflux disease to the Health Insurance Policy Review Committee on February 26, and decided on an internal policy to turn the face-to-face examination of drugs that had been settled through price negotiations.
From this point on, a subsidiary agreement on 'performance of supply obligations' will be drawn up for all drugs undergoing drug price negotiations with the HIRA, and the 'pre-counseling system' will be actively operated until the Minister of Health and Welfare 's order for drug price negotiations.
The HIRA has signed and managed a contract containing the same provisions, including 'Supply Obligations and Patient Protection' on 172 drugs of 59 pharmaceutical companies by October this year.
It is necessary to negotiate in advance whether there is a drug that can be replaced by itself and how the supply will proceed in the future.
In the research process conducted by the HIRA, the foreign drug price standard referred to in the process of listing domestic drug benefits is added from A7 (USA, UK, Germany, France, Italy, Switzerland, Japan) to A10 (additially Taiwan, Canada, Australia).
When the results of the study suggest that they should be expanded.
The movement has begun to improve this.
Recently, the HIRA also added 'Post-Pharmaceutical Evaluation' and 'Herbal Drugs' to the Subsidy Commission of the Pharmaceutical Benefits Evaluation Committee.
The new Sub-Committee on Subsequent Drug Evaluation will conduct post-evaluation of those drugs that have extended their coverage by anticancer drugs, rare drugs, and clinical usefulness, which are expensive drugs.
◆Yang-ho Cho ’s issue=n last July, the prosecution filed a warrant for arrest of the chairman of Hanjin Group, Cho, Yang-ho, for fraud, embezzlement, and duties.
He also illegally borrowed a pharmacist's license and ran a pharmacy to violate the pharmacist law, and retrieval process begins for ₩100 billion in insurance financing.

But, on April 8, when President Cho suddenly passed away, the trial proceedings have been disrupted including violations of the Pharmaceutical Affairs Law.
At present, Mr.
Cho's illegal pharmacy trial continues against Mr.
Won, a pharmacist Lee, and his spouse Mrs Ryu , who conspired to commit the crime.
Sue was suspected of violating pharmacological law by opening a pharmacy on the first floor of the company's annex under the name of pharmacist Lee, through Mr.
Won and Mrs Ryu.
◆Field survey=Since this year, the self-check system has been expanded.
The autonomous check system was introduced to allow the reviewers to detect and report unfair claims by informing the appropriate nursing institution of the possibility of unfairness of the reimbursement expenses already paid before the on-site investigation.
Trial project from December 2017 ▲ Temporary mandibular joint shooting (1st) ▲ Increase of claims after divided use of injection (2nd) ▲ Violation of breast biopsy calculation standard ▲Pharmacy differential index and night addition error claim based on the results of the autonomous inspections in turn, the autonomous inspection system was introduced in earnest from November 1, 2018.
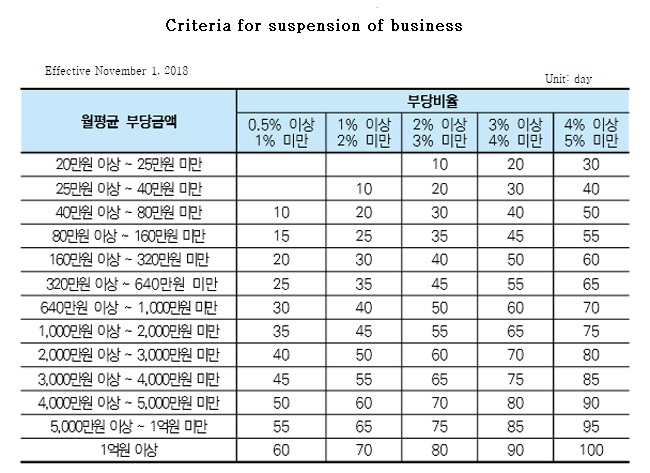
The HIRA feels the necessity of reviewing administrative measures for improvement based on the type and billing amount.
On the other hand, the NHIS has argued that employees of the HIRA should have the special judicial police right to eradicate operated hospitals by office manager, not a doctor.
◆Bending of the first ₩1 trillion in history= The NHIS announced the average rate of increase in the conversion index for nursing institutions on the 1st of next year at 2.29% (bending ₩1.47 trillion).
Although it was determined to be slightly lower than 2019 (2.37%) in consideration of the subscriber's ability, financial integrity, and medical expenses growth rate, it recorded the first ₩1 trillion in sales.
Next year's budget is estimated at ₩1.4688 trillion, with ₩434.9 billion for hospitals, ₩93.5 billion for dentistry, ₩66.9 billion for oriental medicine, and ₩114.2 billion for pharmacies.
The rate of increase was 3.5% in pharmacies, 3.1% in dentistry, 3% in oriental medicine, and 1.7% in hospital, but the order of the distribution of bending shares was reversed.
◆DUR Advancement Pilot Project=The Drug Utilization Review (DUR) Advancement Pilot Project, which has been in progress since August 1st, was completed this month.
The HIRA conducted a pilot project for 20 medical institutions (2 senior general hospitals, 2 general hospitals, 1 hospital, 4 clinics, and 11 pharmacies) in the second half of this year.
Based on the results of 2018 DUR Research Plan for Advancement of DUR, the pilot project was undertaken to establish a comprehensive management system before and after drug use as well as to establish a compensation system.
And there are two types of systems in which pharmacies participate: aftercare, allergy, and adverse event monitoring.
◆Drug serial number system= From January 1, the serial number system has been implemented.
The average reporting rate for the first half of all distributors released in August was 89.1%, at the time of shipment, 2591 companies (96.4%) had a serial number reporting rate of more than 50%, and 98 companies (3.6%) had less than 50%.
The appeals of 18 wholesalers were cited as a result of the request by the HIRA to 98 companies for administrative disposal.
80 unappealed or rejected complaints were selected as the final administrative disposal request companies and notified to the public health center.
In the second half of this year, the administrative reporting criteria for the distribution number serial number of distributors have been raised from 50% to 55%.
From July to December this year, after the first half of this year's accounting period, requests for administrative disposal of manufacturing import companies are applied from the serial number reporting rate in the second half of this year.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









