- LOGIN
- MemberShip
- 2025-12-25 02:52:56
- Moon Care on new drug and generic pricing in 2019
- by Kim, Jung-Ju | translator Byun Kyung A | 2019-12-26 06:29:43

The healthcare coverage enhancement policy lowered the threshold of new drug listing standard, but further complicated the post-marketing drug pricing system.
Also Ministry of Health and Welfare’s (MOHW) had its pharmaceutical benefit sector to concentrate their drug pricing capability on the designing of the technicalities of the healthcare policy.
Lowered threshold, but complicated post-marketing evaluation for all-around management of generic and new drug The impact of the groundbreaking insurance coverage enhancement program by the Moon Care has struck down on the general drug pricing system this year.
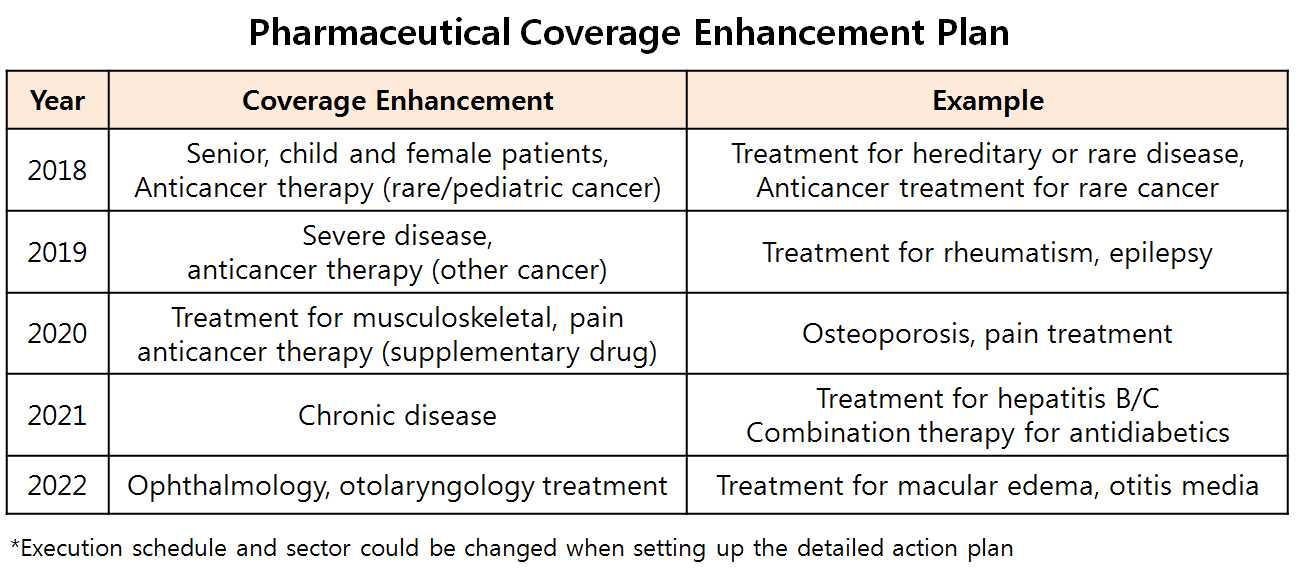
In the beginning of the year, the government, as previously notified, presented generic pricing and new drug listing system revision, listed drug reevaluation and pharmaceutical expense management all at once.
For the bigger frame of enhancing healthcare coverage and reducing the people’s medical bills, the government has decided to progressively reinforce coverage on new drug while strictly managing already-listed new drugs and generics.
In July, MOHW issued an administrative notice on partially revised regulation of ‘Pharmaceutical Affairs Decision Making and Approval Criteria’ with the said changes, and plans to finalize it by the end of the year.
On the other hand, the barrier of new drug listing has been alleviated.
The RSA eligibility and scope have been expanded for high-cost new drugs as constantly demanded by the industry.
But for an item to choose the option, it has to pass the three following conditions; a drug used for treating cancer or disease either recognized by ‘Special Case Standard for Partial Copayment Benefit’ or ‘Special Case Benefit for Patients with Rare Disease and Chronic Disease’; drug with clinical efficacy proven to improve quality of life or recognized so by a related committee; drug recognized as Breakthrough Therapy Designation (BTD) or Priority Medicines (PRIME) by the US Food and Drug Administration (FDA) or European Medicines Agency (EMA), respectively, or recognized as equivalent by Drug Reimbursement Evaluation Committee (DREC).
But some have raised an issue about the regulator turning Health Insurance Policy Deliberation Committee (HIPDC) on-paper reimbursement listing review into a face-to-face review for new drug exempted from negotiation.
They claimed it would cripple the system’s effectiveness and accessibility.
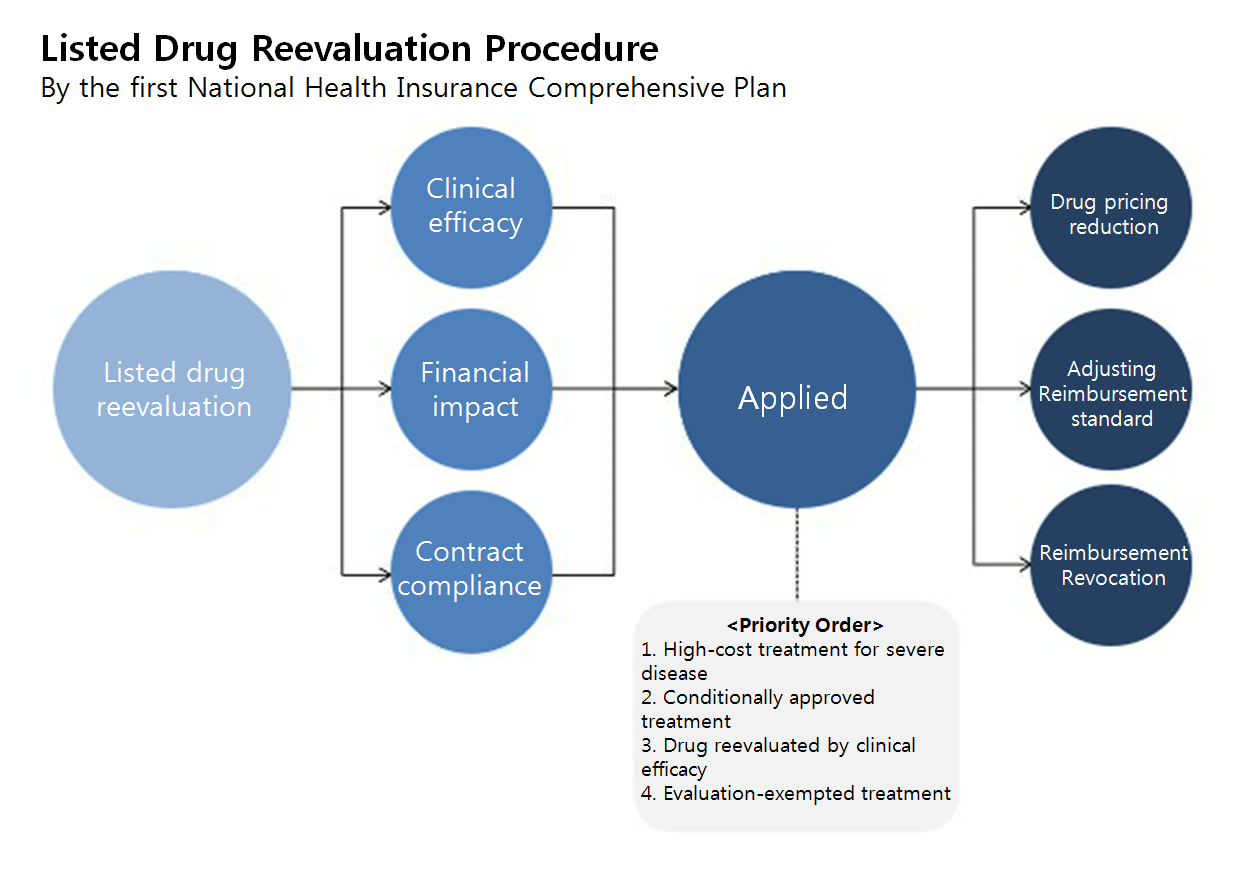
Meanwhile, the government plans to establish a listed drug reevaluation standard and to elaborate the post-marketing evaluation procedure.
The three reevaluation types—external reference pricing, listing contract expired drug, and performance-based post-marketing evaluation—would be categorized by literature-based reevaluation and real world evidence (RWE)-based reevaluation.
But the industry is firmly opposing on the notion of reevaluation, as the result of the reevaluations would eventually either reduce reimbursed price or adjust the general pricing lower.
First it started from the KORUS FTA renegotiation agenda, but technically the government dropped both benefit for Korean-made new drug exporting to global markets and the U.S.-based multinational pharmaceutical companies’ demand for new drug pricing benefit.
Considering the government had its agenda behind it, the industry reprehended the government last year for its concerning and unfair action.
The Drug Pricing Benefit for companies states the manufacturer and suppliers of WHO-recommended essential drug or National Essential Drug as designated by Article 2 of the Pharmaceutical Affairs Act should be confirmed to manufacture and supply without an issue.
And the government would strip the pricing benefit of the companies, if they have issues supplying drugs on the Reimbursed Drug List, or were imposed with administrative penalty or convicted by a court for providing illegal rebate, according to Paragraph 2 of Article 47 of the Pharmaceutical Affairs Act.
But there are exceptions.
A company that suspends the supply of the drug with following reasons would be exempted from the penalty; in case manufacturing plant is shut down or closed; manufacturing, import or sales approval is suspended or canceled; new issue of safety or effectiveness arises; manufactured or imported supply shortage occurs due to surged demand (except when the demanded amount is within the predicted billing amount); when the company is faced with inevitable natural disaster.
The government is also continuing on with the rebate prevention policy.
The so-called ‘K-Sunshine Act’ was enforced and required pharmaceutical companies to file, archive and submit financial profit provision record (within the allowed amount by Pharmaceutical Affairs Act), or the expenditure report.
At the moment, the government is reviewing the submitted expenditure reports.
The government is focusing on analyzing and investigating expenditure types to set the system down.
However, the companies may undergo investigation by prosecution when correlation between their expenditure and rebate are clearly found.
The insurance coverage enhancement policy implemented this year constructed with substance-by-substance reimbursement, expanded listing of high-cost drug, and listed drug reevaluation would base even more specified drug pricing policy for next year.
The industry is expected to see the effect in the field.
Moon Care ploughs on with coverage enhancement with selective reimbursement This year was the first year for the first National Health Insurance Comprehensive Plan.
Last year the government was sketching out the technicality of the Moon Care, and this year it was busy executing the detailed policy actions in the set order.
Starting from the first pilot program of primary healthcare-based chronic disease management (initiated from December 2018), the government gradually increased healthcare coverage on ultrasound scan, essential check up and treatment for lower abdomen (rectums and anal passage) and urinary system (kidney and bladder).
Moreover, coverage on cavity treatment for children under 12, thoracoabdominal MRI scan and Korean medicine treatment has been granted this year.
In addition, the government started the pilot program for Community Care that integrated healthcare and welfare, providing customized benefit by each region across the country.
But with the figure, the public is skeptical about the government raising the National Health Insurance premium by 3.2 percent.
The skepticism is not only about the premium increase, but also about ultimately reducing the people’s medical bills by fixing insurance income source and increasing rate of the government funding.
Both industrial organizations and civic groups are urging the National Assembly to push up the government funding rate up to 20 percent to keep the moderate balance of medical service fee and coverage rate.
Currently, Ministry of Economy and Finance has promised MOHW to provide the government insurance funding of 14 percent for next year, which MOHW would utilize on reinforcing insurance coverage programs.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









