- LOGIN
- MemberShip
- 2025-12-25 02:52:57
- HIRA unveils listed drug reevaluation guideline
- by Kim, Jung-Ju | translator Byun Kyung A | 2019-12-05 06:14:52

The scope of listed drug reevaluation mainly centers expensive drug items, such as anticancer and rare disease treatments, covered by insurance benefit despite their uncertainty in clinical efficacy.
The evaluation borrows the previous reimbursed drug list adjustment procedure, but the criteria are to be set in more intricately to cover different kinds of pharmaceutical benefit provided now.
Health Insurance Review and Assessment Service’ (HIRA) Chief Park Eun-Young of Pharmaceutical Evaluation Improvement Team under Pharmaceutical Department presented a post-marketing reevaluation plan on listed drug at a public hearing convened for the topic on Dec.
3 at the Ferrum Tower, Seoul.
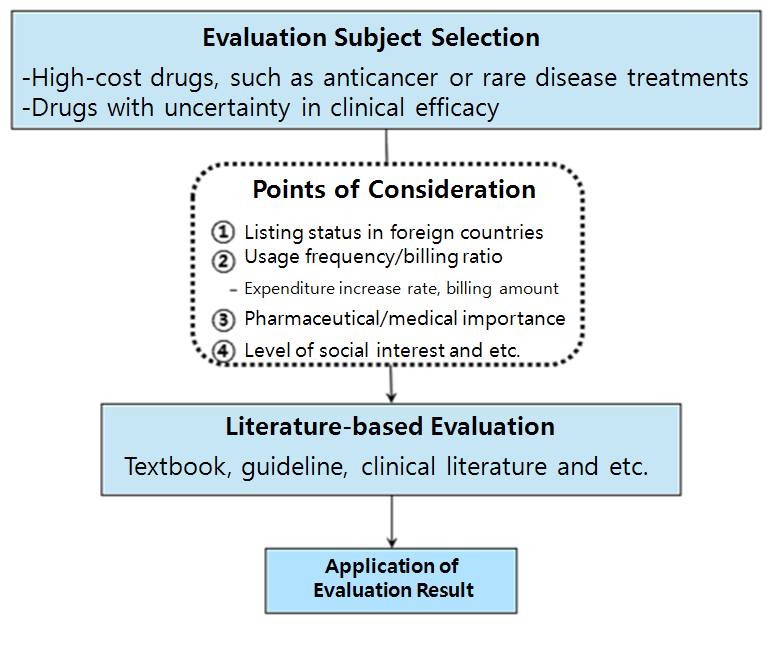
For the reevaluation, applicable items are to be selected from the pool of high-cost drugs treating cancer and rare disease with uncertainty in clinical efficacy.
The selection procedure would take account of item’s insurance listing status in foreign countries, usage frequency, insurance billing ratio, namely pharmaceutical expenditure increase rate and billing amount.
Also levels of pharmaceutical and medical importance and social interest could also affect the procedure.
Initially selected list of drugs would then be evaluated by published literature, such as textbooks, guidelines and clinical literatures.
Basically, the structure of the listed-drug post-evaluation is to follow the footsteps of the Moon Jae-in Care.
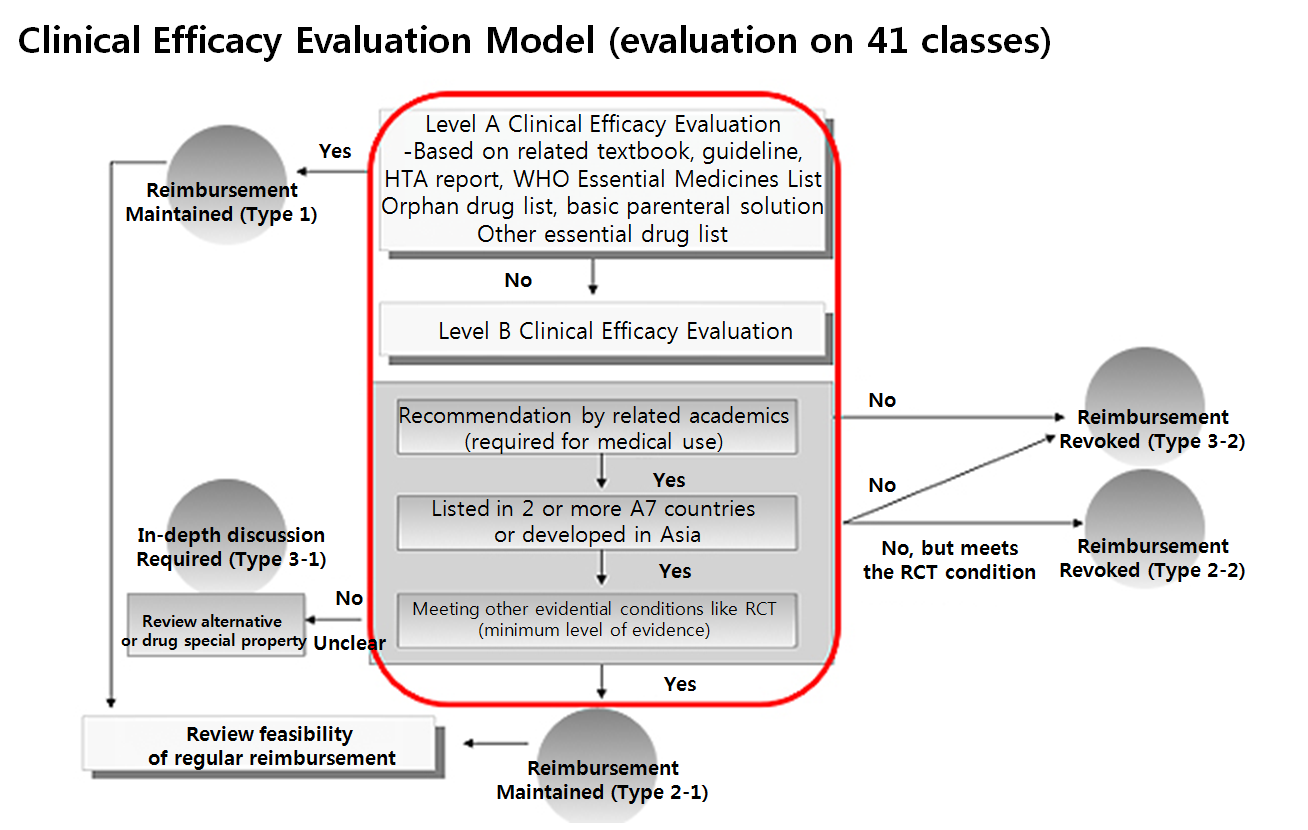
Enforced from 2007 to 2011, the list adjustment procedure also made decisions on reimbursement listing cancellation or restriction based on literature review of related textbooks and guidelines, and verification of medically essential substances.
The Level A clinical efficacy evaluation indicated an item is “clinically effective”, as long as it met at least one of criteria, including Health Technology Assessment (HTA), World Health Organization (WHO) Model List of Essential Medicines (EML), shortage prevention drug, orphan drug, basic parenteral solution, and other essential drug.
Whereas, Level B evaluation indicated an item as “clinically effective” when it met all three criteria of confirming essential medicine recommendation by related academics, recognition by related committees, and usage status in foreign countries.
In the past, the Korean government used to refer to the U.S.
the U.K., France, Italy, Japan, Germany and Switzerland, also known as A7, for the status of reimbursement listing.
For the evaluation, an item had to be listed in more than two countries from the list.
A new drug developed from Korea or in Asia was considered ‘listed in two or more A7 countries.’ And now the government plans to apply the past experience in the reimbursed drug list adjustment procedure on to the new listed drug post-management.
Considering the diversified pharmaceutical benefits since then, the new post-management system is expected to be segmentalized and tightened even more.
Further selection criteria consist of substances requiring clinical efficacy validation by reevaluating effectiveness, requiring tightened management due to change in population structure and increased usage volume, and requiring Post-marketing Drug Reevaluation Subcommittee’s evaluation considering impacts on the society and other healthcare issues.
After the first phase of selection, HIRA would review foreign countries’ approval and reimbursement listing status on the subjects.
Besides the list of seven foreign countries used for the past reimbursed drug list adjustment, Canada is newly added.
Moreover, drug reimbursement billing amount, increased rate claims, billing frequency and its ratio are to be reviewed all around.
Other factors like levels of pharmaceutical and medical importance and social interest would be added as the evaluation criteria.
HIRA would then evaluate the filtered subjects with evenly chosen clinical literatures, such as related textbook, guidelines and HTA reports.
The agency explained alternative treatment options and special property of substances would be considered as well.
When choosing the textbooks and guidelines for the review, HIRA would extensively consider literature’s evidence basis, popularity, expertise, validity, publication period, language, and recognition based on academic society’s evidence evaluation.
Currently, the government and HIRA have not finalized the detailed list of literatures, such as basic list of textbooks from major search database, list of basic guideline from foreign guideline search database, government-related or not-for-profit performance assessment report, and list of published Cochran Reviews and HTA report.
In addition, HIRA is contemplating on taking account of substance demand from related academic societies, alternative feasibility based on available option with equivalent or different mechanisms, and special property of substance.
Restrictive use for pediatric patient, treatment for special patients with HIV-like conditions, and emergent medicine are categorized as special property of substance.
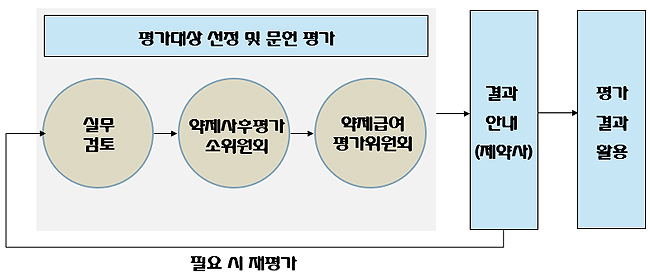
The literature-based evaluation would follow the selection, and then the government agency is to officially notify respective pharmaceutical companies.
The reevaluation can be repeated from the top, if need be, and the result is finalized after the second around.
The government plans to subdivide the reevaluation procedure into finance-based and performance-based post-marketing evaluation, and further enhance the reevaluation procedure.

-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









